...but here are the rules, as passed on by
Greg Laden:
Don't take too long to think about it. Fifteen books you've read that will always stick with you.
First 15 you can recall in no more than 15 minutes.
Except here's the thing: one of the first books that came to mind was one that I can't even remember the title of. It was a huge book (well, huge to a smallish child) that the local library had when I was about ten, probably on South American wildlife, but I just remember these huge double-page dioramas of such things as South American palaeofauna - litopterns, notoungulates and astrapotheres, oh my! - or army ants on the move. Like I said, wouldn't have a clue what it was called now. I'm not even sure that I knew it at the time - it was just the really big book with the cool pictures of
Macrauchenia. Similar issues of memory surround that big book on birds that I used to spend
hours looking at every time I visited my great-grandmother.
So, if I obmit those ones, here's my list:
1 -
Pride and Prejudice by Jane Austen. Yes, it had to be in there somewhere. And to follow on from an
earlier meme, I can now say that I've read all the Austen novels.
Pride and Prejudice isn't necessarily the best - I'd say that that title probably belongs to
Persuasion, while
Northanger Abbey deserves a lot more credit than it's usually given, but I will admit that
P & P is probably the most memorable.
2 -
Peace on Earth by Stanislaw Lem. It was a toss-up between this one and
Solaris. I suspect that only a small proportion of Lem's books have been translated into English, and those that have been are scattered far and wide and almost impossible to find, but every time you do find one you can feel guaranteed that it's an absolute gem. I'd also recommend
The Cyberiad.
3 -
Slaughterhouse Five by Kurt Vonnegut. Again, with an honourable mention to
Galapagos.
4 -
The Kuia and the Spider by Patricia Grace. I cited this children's book in an
earlier post. What I didn't mention there was that
The Kuia and the Spider was actually a revolutionary experience for me as a young child, and one that I'll confess I didn't appreciate right away - it was the first time I was introduced to the idea that a story could have an
ending without having a
conclusion.
5 -
Titus Groan by Mervyn Peake. I read this book a
lot when I was in high school. It still shows through every time I write something.
6 -
The Old Man and the Sea by Ernest Hemingway. I
hated this book. We had to read it in high school -
and watch the stoopid film adaptation. Bored me to
tears. But you know what - almost everything else we read in high school English is long forgotten, but I don't think I'm ever going to forget Hemingway's musings on the true natures of triumph and futility.
7 -
The Book of Lost Tales by J. R. R. Tolkein. I'm going to say something that will probably result in my geek status being instantly revoked -
Tolkein's not really that great. But there's something about the experience of Tolkein, the whole idea of all the hours of scholarship hidden behind the finished product, that somehow ends up imbuing the whole with something far, far more than the cardboard characters, clunky prose and abominable poetry.
The Book of Lost Tales, a collection of early versions of what would end up becoming
The Silmarillion (and, offhand, written in a more paganistic style than the more Catholic morality of the later work) are something of a glimpse behind the magician's curtain.
And the dragons are mechanical.
8 -
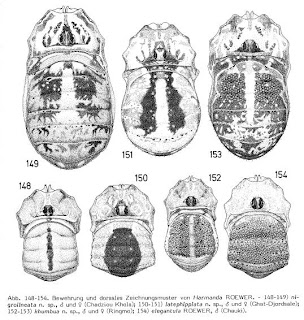
Die Weberknechte der Erde: Systematisches Bearbeitung der Bisher Bekannten Opiliones by Carl-Friedrich Roewer. Again, I'll never forget it for all the wrong reasons. I don't know that I can say I've really
read "Die Weberknechte" - I don't think that even people who actually speak German can understand Roewer. But just try typing "Roewer" into the search box for this blog to get some idea of how much his shadow hangs over my professional career.
9 -
The Hitch-hiker's Guide to the Galaxy by Douglas Adams. What more need I say?
10 -
Perelandra by C. S. Lewis. With the benefit of hindsight and greater maturity, I know that it's as subtle as a half-brick. But some bits of this book genuinely creeped me out when I was a kid.
11 -
Hard-Boiled Wonderland and the End of the World by Haruki Murakami. I got handed this book to read as part of my Japanese Culture class when I was in Fukuoka. I think that by the end of the day, I'd been through it twice. And it's the only work of popular fiction I've known to reference
Synthetoceras and
Cranioceras (even if the process of re-translating a Japanese transliteration of the original names made them all but unrecognisable - I think
Cranioceras became "kuranokeras").
12 -
Alice Through the Looking-Glass by Lewis Carroll. Together with
Alice's Adventures in Wonderland, and bits (but not all) of the
Sylvie and Bruno books, I couldn't get enough Carroll.
13 -
Paradise Lost by John Milton. You always remember
Paradise Lost. Milton later tried to remove the Devil's triumph by writing
Paradise Regained. He failed.
14 -
The Blue Mountain by Meir Shalev. Are the stories true? Are they lies? Does it really matter?
15 -
The Just-So Stories by Rudyard Kipling. Finally, one of the most memorable of all. "He was wild and he was woolly, and his pride was inordinate, and he danced on an outcrop in the middle of Australia". "I am the cat who walks by himself, and all places are alike to me". "The great grey-green Limpopo river, all set about with fever trees". The inimatable rhythm of Kipling's stories is truly unforgettable.