I'm moving house.
Over the past few years, Blogger has become somewhat less user-friendly behind the scenes. Nothing major, and certainly nothing I'm going to bore you with here, but enough that I've finally decided to take the step of breaking out and moving to my own site: varietyoflife.net. This site will incorporate material from both Catalogue of Organisms and Variety of Life, progressively merging them into a single guide to global biodiversity. Posts from both blogs have already been migrated over, though now I have the long task of editing and updating them to match the new format. In the meantime, check out the page on Prostigmata to get an idea of what I'm planning to do. New content will also be appearing regularly.
Thank you for reading Catalogue of Organisms, and I hope to see you in the new digs!
- Home
- Angry by Choice
- Catalogue of Organisms
- Chinleana
- Doc Madhattan
- Games with Words
- Genomics, Medicine, and Pseudoscience
- History of Geology
- Moss Plants and More
- Pleiotropy
- Plektix
- RRResearch
- Skeptic Wonder
- The Culture of Chemistry
- The Curious Wavefunction
- The Phytophactor
- The View from a Microbiologist
- Variety of Life
Field of Science
-
-
Change of address7 months ago in Variety of Life
-
Change of address7 months ago in Catalogue of Organisms
-
-
Earth Day: Pogo and our responsibility10 months ago in Doc Madhattan
-
What I Read 202411 months ago in Angry by Choice
-
I've moved to Substack. Come join me there.1 year ago in Genomics, Medicine, and Pseudoscience
-
-
-
-
Histological Evidence of Trauma in Dicynodont Tusks7 years ago in Chinleana
-
Posted: July 21, 2018 at 03:03PM7 years ago in Field Notes
-
Why doesn't all the GTA get taken up?7 years ago in RRResearch
-
-
Harnessing innate immunity to cure HIV9 years ago in Rule of 6ix
-
-
-
-
-
-
post doc job opportunity on ribosome biochemistry!11 years ago in Protein Evolution and Other Musings
-
Blogging Microbes- Communicating Microbiology to Netizens11 years ago in Memoirs of a Defective Brain
-
Re-Blog: June Was 6th Warmest Globally11 years ago in The View from a Microbiologist
-
-
-
The Lure of the Obscure? Guest Post by Frank Stahl13 years ago in Sex, Genes & Evolution
-
-
Lab Rat Moving House14 years ago in Life of a Lab Rat
-
Goodbye FoS, thanks for all the laughs14 years ago in Disease Prone
-
-
Slideshow of NASA's Stardust-NExT Mission Comet Tempel 1 Flyby15 years ago in The Large Picture Blog
-
in The Biology Files
Bouncing Snail-y Clams
For the most part, bivalves are a fairly conservative bunch. They seem to have worked out what they are good at early on in their history and most of them stick to it. There are, however, notable exceptions and perhaps few groups of bivalves are as exceptional as the Galeommatidae.
Waldo paucitentaculatus, from Valentich-Scott et al. (2013).
Galeommatids are small bivalves, less than a centimetre in length, with more or less thin shells. The hinge teeth are generally weak or absent. The valves of the shell are more or less gaping and in life are at least partially covered by the large, reflected mantle. The outer surface of the mantle is warty and bears several slender tentacles, the exact arrangement of tentacles varying by species. The foot is large and extends well outwards from the central body of the animal. In the most extreme cases, you might be forgiven for thinking you were looking at some sort of snail rather than a clam.
Many galeommatids have been found living as symbionts with other invertebrates such as in the burrows of annelids and crustaceans, or crawling on the surface of echinoderms. So far as is known, these relationships are commensal only, the clams using their hosts as a source of shelter and possibly excess food scraps, but species may be very exclusive in their choice of hosts. For instance, Mikkelsen & Bieler (1989) found the species Divariscintilla yoyo and D. troglodytes only in burrows of the mantis shrimp Lysiosquilla scabricauda, never in burrows of other potential hosts in the same area. It seems likely that this commensalism has allowed galeommatids to diversify in soft-bottom habitats, their larger hosts being able to dig into sediments in which the smaller clams would be quickly smothered (Valentich-Scott et al. 2013).
Unidentified galeommatid, copyright Ria Tan.
Many galeommatids possess a distinctive 'hanging-foot' morphology with the foot divided into two sections, a muscular anterior portion adapted for snail-like crawling, and an elastic posterior section (Bieler & Mikkelsen 1992). The primary byssus gland is located in the anterior section and is connected by a ciliated ventral groove to a terminal adhesive gland in the posterior section. Mikkelsen & Bieler (1989) found that Divariscintilla individuals kept in an aquarium spent most of their time hanging suspended via the posterior part of the foot. Threads produced by the byssus gland were transferred to the terminal adhesor and used to attach to a surface such as the glass of the aquarium (presumably, the clams would normally hang in this manner on the interior wall of the host burrow). When disturbed, hanging clams would rapidly bounce themselves up and down from their attachment point (hence one species being dubbed 'Divariscintilla yoyo'). If the clams wished to change their location, they would crawl on the muscular section of the foot, breaking the byssus threads behind them. The elastic part of the foot was not functional in crawling.
The majority of galeommatid clams are hermaphrodites, either protandrous (beginning life as males before maturing into females) or simultaneous. Eggs are not released into the water column but brooded within the ctenidia until larvae are released at a relatively advanced stage of development (whether the parent is able to feed while its gills are so occupied, I don't know). In a number of species, dwarf males are also present that do not live independently but reside within the mantle cavity of a female (I have seen these males referred to as 'parasitic' but I do not know if they are directly so). In this position, they are able to fertilise the female directly. Such behaviour may be seen as a further adaptation to the clam's commensal lifestyle, contained within the burrow of its host and potentially secluded from more conventional mates. Hidden away in the darkness, they make matryoshkas of themselves.
REFERENCES
Bieler, R., & P. M. Mikkelsen. 1992. Preliminary phylogenetic analysis of the bivalve family Galeommatidae. American Malacological Bulletin 9 (2): 157–164.
Mikkelsen, P. M., & R. Bieler. 1989. Biology and comparative anatomy of Divariscintilla yoyo and D. troglodytes, two new species of Galeommatidae (Bivalvia) from stomatopod burrows in eastern Florida. Malacologia 31 (1): 175–195.
Valentich-Scott, P., D. Ó. Foighil & J. Li. 2013. Where's Waldo? A new commensal species, Waldo arthuri (Mollusca, Bivalvia, Galeommatidae) from the northeastern Pacific Ocean. ZooKeys 316: 67–80.
Galeommatids are small bivalves, less than a centimetre in length, with more or less thin shells. The hinge teeth are generally weak or absent. The valves of the shell are more or less gaping and in life are at least partially covered by the large, reflected mantle. The outer surface of the mantle is warty and bears several slender tentacles, the exact arrangement of tentacles varying by species. The foot is large and extends well outwards from the central body of the animal. In the most extreme cases, you might be forgiven for thinking you were looking at some sort of snail rather than a clam.
Many galeommatids have been found living as symbionts with other invertebrates such as in the burrows of annelids and crustaceans, or crawling on the surface of echinoderms. So far as is known, these relationships are commensal only, the clams using their hosts as a source of shelter and possibly excess food scraps, but species may be very exclusive in their choice of hosts. For instance, Mikkelsen & Bieler (1989) found the species Divariscintilla yoyo and D. troglodytes only in burrows of the mantis shrimp Lysiosquilla scabricauda, never in burrows of other potential hosts in the same area. It seems likely that this commensalism has allowed galeommatids to diversify in soft-bottom habitats, their larger hosts being able to dig into sediments in which the smaller clams would be quickly smothered (Valentich-Scott et al. 2013).
Many galeommatids possess a distinctive 'hanging-foot' morphology with the foot divided into two sections, a muscular anterior portion adapted for snail-like crawling, and an elastic posterior section (Bieler & Mikkelsen 1992). The primary byssus gland is located in the anterior section and is connected by a ciliated ventral groove to a terminal adhesive gland in the posterior section. Mikkelsen & Bieler (1989) found that Divariscintilla individuals kept in an aquarium spent most of their time hanging suspended via the posterior part of the foot. Threads produced by the byssus gland were transferred to the terminal adhesor and used to attach to a surface such as the glass of the aquarium (presumably, the clams would normally hang in this manner on the interior wall of the host burrow). When disturbed, hanging clams would rapidly bounce themselves up and down from their attachment point (hence one species being dubbed 'Divariscintilla yoyo'). If the clams wished to change their location, they would crawl on the muscular section of the foot, breaking the byssus threads behind them. The elastic part of the foot was not functional in crawling.
The majority of galeommatid clams are hermaphrodites, either protandrous (beginning life as males before maturing into females) or simultaneous. Eggs are not released into the water column but brooded within the ctenidia until larvae are released at a relatively advanced stage of development (whether the parent is able to feed while its gills are so occupied, I don't know). In a number of species, dwarf males are also present that do not live independently but reside within the mantle cavity of a female (I have seen these males referred to as 'parasitic' but I do not know if they are directly so). In this position, they are able to fertilise the female directly. Such behaviour may be seen as a further adaptation to the clam's commensal lifestyle, contained within the burrow of its host and potentially secluded from more conventional mates. Hidden away in the darkness, they make matryoshkas of themselves.
REFERENCES
Bieler, R., & P. M. Mikkelsen. 1992. Preliminary phylogenetic analysis of the bivalve family Galeommatidae. American Malacological Bulletin 9 (2): 157–164.
Mikkelsen, P. M., & R. Bieler. 1989. Biology and comparative anatomy of Divariscintilla yoyo and D. troglodytes, two new species of Galeommatidae (Bivalvia) from stomatopod burrows in eastern Florida. Malacologia 31 (1): 175–195.
Valentich-Scott, P., D. Ó. Foighil & J. Li. 2013. Where's Waldo? A new commensal species, Waldo arthuri (Mollusca, Bivalvia, Galeommatidae) from the northeastern Pacific Ocean. ZooKeys 316: 67–80.
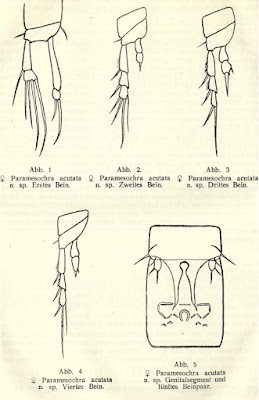
Paramesochra acutata
Copepod taxonomy, it seems, is largely about counting setae. In his review of relationships within the interstitial harpacticoid family Paramesochridae, Huys (1987) recognised four species groups within the genus Paramesochra (which previously got a look-in at this site here). One of these groups, labelled the P. acutata-group, was characterised by reductions in numbers of setae, having lost the inner setae on the first segments of the endopods on the third and fourth legs.
Paramesochra taeana, a close relative of P. acutata, from Back & Lee (2010).
The group takes its name from the species Paramesochra acutata, described by Klie in 1935 from samples taken from coastal groundwater near the town of Schilksee on the northeastern coast of Germany, in the state of Schleswig-Holstein. Other notable features of P. acutata include the presence of four setae on the antennary exopod, well-developed narrow, triangular endopodal lobes on the modified fifth legs of the females, and conical caudal rami produced into spinose processes (Back & Lee 2013). I haven't been able to find whether P. acutata has been collected much beyond its initial locality but other members of its species group have been found around the world. One of these, P. hawaiensis, from (nach) Hawaii, is similar enough that it was until recently treated as a subspecies of P. acutata.
Appendages of female Paramesochra acutata, from Klie (1935).
So what, if anything, does all this mean? That, I'm afraid, is getting a bit beyond me. The fifth legs are used in spermatophore transfer and differences between species might presumably function in recognising suitable mates. Regarding the details of setation and ramus appearance, one wonders if there could be any relation to preferred micro-habitat. Are harpacticoids with fewer setae and more robust rami adapted for crawling among coarser sand grains? Honestly, I have no idea. Anyone care to find out?
REFERENCES
Back, J., & W. Lee. 2010. A new species of the genus Paramesochra (Copepoda: Harpacticoida) from Korean waters. Proceedings of the Biological Society of Washington 123 (1): 47–61.
Back, J., & W. Lee. 2013. Three new species of the genus Paramesochra T. Scott, 1892 (Copepoda: Harpacticoida: Paramesochridae) from Yellow Sea, Korea with a redescription of Paramesochra similis Kunz, 1936. Journal of Natural History 47 (5–12): 769–803.
Huys, R. 1987. Paramesochra T. Scott, 1892 (Copepoda, Harpacticoida): a revised key, including a new species from the SW Dutch coast and some remarks on the phylogeny of the Paramesochridae. Hydrobiologia 144: 193–210.
Klie, W. 1935. Die Harpacticoiden des Küstengrundwassers bei Schilksee (Kieler Förde). Schriften des Naturwissenschaftlichen Vereins für Schleswig-Holstein 20 (2): 409–421.
The group takes its name from the species Paramesochra acutata, described by Klie in 1935 from samples taken from coastal groundwater near the town of Schilksee on the northeastern coast of Germany, in the state of Schleswig-Holstein. Other notable features of P. acutata include the presence of four setae on the antennary exopod, well-developed narrow, triangular endopodal lobes on the modified fifth legs of the females, and conical caudal rami produced into spinose processes (Back & Lee 2013). I haven't been able to find whether P. acutata has been collected much beyond its initial locality but other members of its species group have been found around the world. One of these, P. hawaiensis, from (nach) Hawaii, is similar enough that it was until recently treated as a subspecies of P. acutata.
So what, if anything, does all this mean? That, I'm afraid, is getting a bit beyond me. The fifth legs are used in spermatophore transfer and differences between species might presumably function in recognising suitable mates. Regarding the details of setation and ramus appearance, one wonders if there could be any relation to preferred micro-habitat. Are harpacticoids with fewer setae and more robust rami adapted for crawling among coarser sand grains? Honestly, I have no idea. Anyone care to find out?
REFERENCES
Back, J., & W. Lee. 2010. A new species of the genus Paramesochra (Copepoda: Harpacticoida) from Korean waters. Proceedings of the Biological Society of Washington 123 (1): 47–61.
Back, J., & W. Lee. 2013. Three new species of the genus Paramesochra T. Scott, 1892 (Copepoda: Harpacticoida: Paramesochridae) from Yellow Sea, Korea with a redescription of Paramesochra similis Kunz, 1936. Journal of Natural History 47 (5–12): 769–803.
Huys, R. 1987. Paramesochra T. Scott, 1892 (Copepoda, Harpacticoida): a revised key, including a new species from the SW Dutch coast and some remarks on the phylogeny of the Paramesochridae. Hydrobiologia 144: 193–210.
Klie, W. 1935. Die Harpacticoiden des Küstengrundwassers bei Schilksee (Kieler Förde). Schriften des Naturwissenschaftlichen Vereins für Schleswig-Holstein 20 (2): 409–421.
Melanterius Weevils
Here in the Antipodes, we have a long history of environmental upheaval from exotic taxa unwisely released. As a result, one can't help but feel an odd twinge of perverse patriotism when hearing of the inverse, some native of the Antipodes causing grief elsewhere. In South Africa, Australian acacias have become something of an issue, inciting a search for potential control agents. Among the candidates selected are weevils of the genus Melanterius.
Melanterius servulus, copyright Sally Adam.
Melanterius is a diverse genus of small black or brown weevils (ranging from about three to seven millimetres in length) that feed as both adults and larvae on the developing seeds of acacias. About eighty species have been recognised in the genus to date and possibly many more remain to be described. In general, Melanterius weevils are heavily punctate, usually without prominent hairs but with a covering of scales. The rostrum is reasonably long, reaching more or less back to the mesosternum at rest but not sitting in a distinct ventral groove, and may be variably curved (going by figures in Zimmerman 1992).
Melanterius semiporcatus, copyright Victor W. Fazio III.
As with other weevils, the prominent rostrum is used by females to chew into an appropriate spot on the host plant, in this case chewing holes into the developing acacia seed pods, into which eggs are laid. Melanterius species go through one generation per year. Larvae burrow into and feed on the developing seeds before emerging and dropping to the ground to pupate in the soil. Mature adults emerge well before the host acacias begin to set seeds, usually having to wait about six months (Auld 1989). They usually spend the intervening period largely inactive, sheltering in concealed places close to the host plant and occasionally emerging to briefly feed on developing buds.
Under peak conditions, Melanterius infestations may cause a complete failure of seed production. No wonder, then, that they have been considered a worthwhile instrument of biological control.
REFERENCES
Auld, T. D. 1989. Larval survival in the soil and adult emergence in Melanterius Erichson and Plaesiorhinus Blackburn (Coleoptera: Curculionidae) following seed feeding on Acacia and Bossiaea (Fabaceae). Journal of the Australian Entomological Society 28: 235–238.
Zimmerman, E. C. 1992. Australian Weevils (Coleoptera: Curculionoidea) vol. 6. Colour plates 305–632. CSIRO Australia.
Melanterius is a diverse genus of small black or brown weevils (ranging from about three to seven millimetres in length) that feed as both adults and larvae on the developing seeds of acacias. About eighty species have been recognised in the genus to date and possibly many more remain to be described. In general, Melanterius weevils are heavily punctate, usually without prominent hairs but with a covering of scales. The rostrum is reasonably long, reaching more or less back to the mesosternum at rest but not sitting in a distinct ventral groove, and may be variably curved (going by figures in Zimmerman 1992).
As with other weevils, the prominent rostrum is used by females to chew into an appropriate spot on the host plant, in this case chewing holes into the developing acacia seed pods, into which eggs are laid. Melanterius species go through one generation per year. Larvae burrow into and feed on the developing seeds before emerging and dropping to the ground to pupate in the soil. Mature adults emerge well before the host acacias begin to set seeds, usually having to wait about six months (Auld 1989). They usually spend the intervening period largely inactive, sheltering in concealed places close to the host plant and occasionally emerging to briefly feed on developing buds.
Under peak conditions, Melanterius infestations may cause a complete failure of seed production. No wonder, then, that they have been considered a worthwhile instrument of biological control.
REFERENCES
Auld, T. D. 1989. Larval survival in the soil and adult emergence in Melanterius Erichson and Plaesiorhinus Blackburn (Coleoptera: Curculionidae) following seed feeding on Acacia and Bossiaea (Fabaceae). Journal of the Australian Entomological Society 28: 235–238.
Zimmerman, E. C. 1992. Australian Weevils (Coleoptera: Curculionoidea) vol. 6. Colour plates 305–632. CSIRO Australia.
Kirkby's Small Ostracods (or Small Kirkby's Ostracods)
I do not envy those who find themselves working with ostracods. These minute crustaceans, typically less than a millimetre in length, seem altogether too fiddly to handle. Nevertheless, the long history of ostracods, together with their diversity and the high fossilisation potential of their calcified carapace valves, have made them a common focus for studying biostratigraphy and historical environments. The classification of modern ostracods is commonly informed by features of the legs and other appendages but such characters are not commonly preserved in fossil representatives. As a result, there are many groups of ostracods known from the Palaeozoic whose relationships remain uncertain.
Left valve of Kirkbyella delicata, from Hoare & Merrill (2004).
One such group is classified by Liebau (2005) as the superfamily Kirkbyelloidea. Members of this group are small ostracods with reticulate valves. The dorsal and ventral margins of the valves tend to be more or less straight. They are commonly impressed with a single dorsal sulcus, extending downwards from the dorsal margin about halfway along the valve's length. Below this sulcus is a protruding horizontal lobe ending in members of the family Kirkbyellidae in a small spine. Evidence of sexual dimorphism, a not-uncommon feature of Palaeozoic ostracods, is not known from kirkbyelloids.
Definite kirkbyelloids are known from the Devonian to the Permian. If the earlier family Ordovizonidae is included, their record extends all the way back to the Ordovician. As noted above, it is unclear where kirkbyelloids sit in the ostracod family tree. Becker (1994) suggested a relationship via Ordovizona to the Ordovician Monotiopleuridae which resemble kirkbyelloids in the outline of the carapace valves and features of the adductor muscle scars. Though long-lived, kirkbyelloids don't seem to have ever been massively diverse, and they can probably be counted among the many lineages of organisms that never made it past the end of the Palaeozoic.
REFERENCES
Becker, G. 1994. A remarkable Ordovician ostracod fauna from Orphan Knoll, Labrador Sea. Scripta Geologica 107: 1–25.
Hoare, R. D., & G. K. Merrill. 2004. A Pennsylvanian (Morrowan) ostracode fauna from Texas. Journal of Paleontology 78 (1): 185–204.
Liebau, A. 2005. A revised classification of the higher taxa of the Ostracoda (Crustacea). Hydrobiologia 538: 115–137.
One such group is classified by Liebau (2005) as the superfamily Kirkbyelloidea. Members of this group are small ostracods with reticulate valves. The dorsal and ventral margins of the valves tend to be more or less straight. They are commonly impressed with a single dorsal sulcus, extending downwards from the dorsal margin about halfway along the valve's length. Below this sulcus is a protruding horizontal lobe ending in members of the family Kirkbyellidae in a small spine. Evidence of sexual dimorphism, a not-uncommon feature of Palaeozoic ostracods, is not known from kirkbyelloids.
Definite kirkbyelloids are known from the Devonian to the Permian. If the earlier family Ordovizonidae is included, their record extends all the way back to the Ordovician. As noted above, it is unclear where kirkbyelloids sit in the ostracod family tree. Becker (1994) suggested a relationship via Ordovizona to the Ordovician Monotiopleuridae which resemble kirkbyelloids in the outline of the carapace valves and features of the adductor muscle scars. Though long-lived, kirkbyelloids don't seem to have ever been massively diverse, and they can probably be counted among the many lineages of organisms that never made it past the end of the Palaeozoic.
REFERENCES
Becker, G. 1994. A remarkable Ordovician ostracod fauna from Orphan Knoll, Labrador Sea. Scripta Geologica 107: 1–25.
Hoare, R. D., & G. K. Merrill. 2004. A Pennsylvanian (Morrowan) ostracode fauna from Texas. Journal of Paleontology 78 (1): 185–204.
Liebau, A. 2005. A revised classification of the higher taxa of the Ostracoda (Crustacea). Hydrobiologia 538: 115–137.
Conformed Flycatchers
A quote I have often had cause to refer to—I believe it originally came from Toby White of Palaeos.com—is that "organisms are under no obligation to speciate with regard to the convenience of taxonomists". For birdwatchers in North America, perhaps no group more embodies this principle than the flycatchers of the genus Empidonax. These small members of the hyperdiverse New World family Tyrannidae comprise fifteen recognised species that have become notorious for the difficulty in telling them apart.
Immature alder flycatcher Empidonax alnorum, copyright Cephas.
The species of Empidonax are uniformly olive brown above, lighter below, with pale rings around the eyes and bands on the wings. They are inhabitants of woodlands (more on that in a moment) and watch for flying insects from a perch, making short flights to capture prey. Though individual species are generally similar in their feeding habits, they are often specifically distinct in their preferred habitats. A molecular (mtDNA) analysis of Empidonax species by Johnson & Cicero (2002) identified four likely clades within the genus with members of a clade each differing in their specific breeding range. Species found in the US and Canada often migrate long distances and closely related species may be found close together outside their breeding ranges (references to ranges below refer to breeding ranges). Species found in Mexico and Central America are more likely to migrate only short distances or be resident year-round.
Acadian flycatcher Empidonax virescens, copyright Aitor.
The Acadian flycatcher E. virescens seems to be relatively isolated from other members of the genus. This species is found in shady forests near water in the eastern US and Canada. Its nest is a cup made from plant fibres suspended in a horizontal branch fork, and it lays lightly speckled eggs.
The yellow-bellied flycatcher E. flaviventris, yellowish flycatcher E. flavescens, Cordilleran flycatcher E. occidentalis and Pacific slope flycatcher E. difficilis form a clade of species that tend to have more yellowish underparts than other members of the genus. Their nests are mossy cups constructed on a protected ledge or crevice. Members of this clade tend to be found in relatively damp forest areas, such as boggy areas of boreal forests in the case of E. flaviventris, or shady canyons in the case of E. occidentalis or E. difficilis. A notable exception is the Channel Islands population of E. difficilis which is found in more open woodlands than its mainland counterparts. Empidonax occidentalis and E. difficilis are found in the western United States with E. difficilis occupying coastal regions and E. occidentalis found further inland. Until fairly recently, the two were confused as a single species; they are almost indistinguishable morphologically but can be separated by their calls.
Least flycatcher Empidonax minimus, copyright Mdf.
The white-throated flycatcher E. albigularis, alder flycatcher E. alnorum and willow flycatcher E. traillii form a clade of species nesting in damp thickets. Again, it was only fairly recently that the more northerly E. alnorum was distinguished from the more southerly E. traillii.
Finally, the remaining species form a clade whose members lay eggs without speckled markings. They are often relatively dark compared to other Empidonax; the black-capped flycatcher E. atriceps of Costa Rica and Panama stands out for the sooty-black coloration of the head. They often inhabit relatively open forest, often at higher altitudes.
Johnson & Cicero (2002) suggested that the largely allopatric (non-overlapping) breeding ranges of species within clades of Empidonax reflected speciation as a result of isolation in glacial refuges during the ice ages. As the ice retreated, the now-distinct species expanded their ranges but excluded each other where they met. Differences in mating calls between related species dissuaded interbreeding. Physical appearance, meanwhile, remained frustratingly monotonous.
REFERENCE
Johnson, N. K., & C. Cicero. 2002. The role of ecologic diversification in sibling speciation of Empidonax flycatchers (Tyrannidae): multigene evidence from mtDNA. Molecular Ecology 11: 2065–2081.
The species of Empidonax are uniformly olive brown above, lighter below, with pale rings around the eyes and bands on the wings. They are inhabitants of woodlands (more on that in a moment) and watch for flying insects from a perch, making short flights to capture prey. Though individual species are generally similar in their feeding habits, they are often specifically distinct in their preferred habitats. A molecular (mtDNA) analysis of Empidonax species by Johnson & Cicero (2002) identified four likely clades within the genus with members of a clade each differing in their specific breeding range. Species found in the US and Canada often migrate long distances and closely related species may be found close together outside their breeding ranges (references to ranges below refer to breeding ranges). Species found in Mexico and Central America are more likely to migrate only short distances or be resident year-round.
The Acadian flycatcher E. virescens seems to be relatively isolated from other members of the genus. This species is found in shady forests near water in the eastern US and Canada. Its nest is a cup made from plant fibres suspended in a horizontal branch fork, and it lays lightly speckled eggs.
The yellow-bellied flycatcher E. flaviventris, yellowish flycatcher E. flavescens, Cordilleran flycatcher E. occidentalis and Pacific slope flycatcher E. difficilis form a clade of species that tend to have more yellowish underparts than other members of the genus. Their nests are mossy cups constructed on a protected ledge or crevice. Members of this clade tend to be found in relatively damp forest areas, such as boggy areas of boreal forests in the case of E. flaviventris, or shady canyons in the case of E. occidentalis or E. difficilis. A notable exception is the Channel Islands population of E. difficilis which is found in more open woodlands than its mainland counterparts. Empidonax occidentalis and E. difficilis are found in the western United States with E. difficilis occupying coastal regions and E. occidentalis found further inland. Until fairly recently, the two were confused as a single species; they are almost indistinguishable morphologically but can be separated by their calls.
The white-throated flycatcher E. albigularis, alder flycatcher E. alnorum and willow flycatcher E. traillii form a clade of species nesting in damp thickets. Again, it was only fairly recently that the more northerly E. alnorum was distinguished from the more southerly E. traillii.
Finally, the remaining species form a clade whose members lay eggs without speckled markings. They are often relatively dark compared to other Empidonax; the black-capped flycatcher E. atriceps of Costa Rica and Panama stands out for the sooty-black coloration of the head. They often inhabit relatively open forest, often at higher altitudes.
Johnson & Cicero (2002) suggested that the largely allopatric (non-overlapping) breeding ranges of species within clades of Empidonax reflected speciation as a result of isolation in glacial refuges during the ice ages. As the ice retreated, the now-distinct species expanded their ranges but excluded each other where they met. Differences in mating calls between related species dissuaded interbreeding. Physical appearance, meanwhile, remained frustratingly monotonous.
REFERENCE
Johnson, N. K., & C. Cicero. 2002. The role of ecologic diversification in sibling speciation of Empidonax flycatchers (Tyrannidae): multigene evidence from mtDNA. Molecular Ecology 11: 2065–2081.
The Teleost Fuse
A while back, I discussed the group of fish known as the Holostei, the gars and bowfin. The Holostei constitute one branch of the clade Neopterygii which includes the majority of living ray-finned fishes. However, their success in the modern environment pales in comparison to that of their sister group, the Teleostei.
Siemensichthys macrocephalus, an early teleost of uncertain affinities, copyright Ghedoghedo.
Teleosts are such a major component of ray-finned fishes that it is simpler to list those members of the modern fauna that do not belong to this clade: the aforementioned gars and bowfin, sturgeons and paddlefish, and the bichirs of Africa. Everything else belongs to the great teleost radiation, representing about 96% of all modern fishes. The earliest fishes generally recognised as teleosts come from marine deposits of the Late Triassic in the form of the Pholidophoridae of Europe. The earliest known members of the crown group are from the Late Jurassic (Nelson et al. 2016). Teleosts have been recognised as an apomorphy-defined clade; the crown clade has been dubbed the Teleocephala. Among the features that have been used to define the Teleostei are the presence of a mobile premaxilla. In my previous post, I explained how the mobile maxilla of neopterygians including bowfins improved feeding by creating suction when the mouth was opened. Having both the maxilla and premaxilla mobile enhances this process further. In some of the most advanced teleosts, such as dories and ponyfish, the connection between the jaws and the cranium is entirely comprised of soft, flexible tissue, allowing the jaw apparatus as a whole to be catapulted towards unwary prey. Other features that have been highlighted include a strongly ossified caudal skeleton with long uroneural spines derived from the neural arches of the vertebrae, and the lower lobe of the caudal fin supported by two plate-like hypural bones articulating with a single vertebral centrum (Bond 1996).
Leptolepis coryphaenoides, one of the earliest teleosts with cycloid scales, copyright Daderot.
Of course, not all these features necessarily appeared in lock with each other. A phylogenetic analysis of basal teleosts by Arratia (2013) identified the aforementioned features of the caudal skeleton as absent in some of the basalmost teleosts. The condition of the premaxilla is ambiguous in Prohalecites, the earliest stem-group teleost from the Middle-Late Triassic boundary. It appears to be absent in the Aspidorhynchiformes and Pachycormiformes, Mesozoic orders that are currently regarded as on the teleost stem but not part of the Teleostei. However, as was found with the mobile maxilla in gars, one can't help wondering whether this character has been affected by the uniquely derived upper jaw morphologies in these orders. Other features identified by Arratia (2013) as supporting the Teleostei clade include the presence of two supramaxillary bones, a suborbital bone between the posterior margin of the posterodorsal infraorbitals and the anterior margin of the opercular apparatus (subsequently lost in the teleost crown group), and accessory suborbital bones ventrolateral to the postorbital region of the skull roof.
The earliest teleosts in the Pholidophoridae and other basal lineages retained the heavy ganoid scales of thick bone that may still be seen in modern Teleostei. Lighter, thinner cycloid scales first appear with the Early Jurassic Leptolepis coryphaenoides (Arratia 2013) and are the basal scale type for the teleost crown group (in some derived subgroups, the scales would become further modified or even lost). The greater mobility permitted by these lighter scales may have been another significant factor in the teleost explosion. By the Cretaceous period, stem-teleosts had radiated into a variety of specialised forms such as the gigantic predatory Ichthyodectiformes (of which Xiphactinus grew up to four metres in length) and the deep-finned Araripichthys. The three major subgroups of the crown Teleostei—the Elopomorpha, Osteoglossomorpha and Clupeocephala—had diverged from each other by the end of the Jurassic. The stem-teleosts would disappear with the end of the Mesozoic; the crown teleosts would dominates the world's waters from that time on.
REFERENCES
Arratia, G. 2013. Morphology, taxonomy, and phylogeny of Triassic pholidophorid fishes (Actinopterygii, Teleostei). Journal of Vertebrate Paleontology 33 (6 Suppl.): 1–138.
Nelson, J. S., T. C. Grande & M. V. H. Wilson. 2016. Fishes of the World 5th ed. Wiley.
Teleosts are such a major component of ray-finned fishes that it is simpler to list those members of the modern fauna that do not belong to this clade: the aforementioned gars and bowfin, sturgeons and paddlefish, and the bichirs of Africa. Everything else belongs to the great teleost radiation, representing about 96% of all modern fishes. The earliest fishes generally recognised as teleosts come from marine deposits of the Late Triassic in the form of the Pholidophoridae of Europe. The earliest known members of the crown group are from the Late Jurassic (Nelson et al. 2016). Teleosts have been recognised as an apomorphy-defined clade; the crown clade has been dubbed the Teleocephala. Among the features that have been used to define the Teleostei are the presence of a mobile premaxilla. In my previous post, I explained how the mobile maxilla of neopterygians including bowfins improved feeding by creating suction when the mouth was opened. Having both the maxilla and premaxilla mobile enhances this process further. In some of the most advanced teleosts, such as dories and ponyfish, the connection between the jaws and the cranium is entirely comprised of soft, flexible tissue, allowing the jaw apparatus as a whole to be catapulted towards unwary prey. Other features that have been highlighted include a strongly ossified caudal skeleton with long uroneural spines derived from the neural arches of the vertebrae, and the lower lobe of the caudal fin supported by two plate-like hypural bones articulating with a single vertebral centrum (Bond 1996).
Of course, not all these features necessarily appeared in lock with each other. A phylogenetic analysis of basal teleosts by Arratia (2013) identified the aforementioned features of the caudal skeleton as absent in some of the basalmost teleosts. The condition of the premaxilla is ambiguous in Prohalecites, the earliest stem-group teleost from the Middle-Late Triassic boundary. It appears to be absent in the Aspidorhynchiformes and Pachycormiformes, Mesozoic orders that are currently regarded as on the teleost stem but not part of the Teleostei. However, as was found with the mobile maxilla in gars, one can't help wondering whether this character has been affected by the uniquely derived upper jaw morphologies in these orders. Other features identified by Arratia (2013) as supporting the Teleostei clade include the presence of two supramaxillary bones, a suborbital bone between the posterior margin of the posterodorsal infraorbitals and the anterior margin of the opercular apparatus (subsequently lost in the teleost crown group), and accessory suborbital bones ventrolateral to the postorbital region of the skull roof.
The earliest teleosts in the Pholidophoridae and other basal lineages retained the heavy ganoid scales of thick bone that may still be seen in modern Teleostei. Lighter, thinner cycloid scales first appear with the Early Jurassic Leptolepis coryphaenoides (Arratia 2013) and are the basal scale type for the teleost crown group (in some derived subgroups, the scales would become further modified or even lost). The greater mobility permitted by these lighter scales may have been another significant factor in the teleost explosion. By the Cretaceous period, stem-teleosts had radiated into a variety of specialised forms such as the gigantic predatory Ichthyodectiformes (of which Xiphactinus grew up to four metres in length) and the deep-finned Araripichthys. The three major subgroups of the crown Teleostei—the Elopomorpha, Osteoglossomorpha and Clupeocephala—had diverged from each other by the end of the Jurassic. The stem-teleosts would disappear with the end of the Mesozoic; the crown teleosts would dominates the world's waters from that time on.
REFERENCES
Arratia, G. 2013. Morphology, taxonomy, and phylogeny of Triassic pholidophorid fishes (Actinopterygii, Teleostei). Journal of Vertebrate Paleontology 33 (6 Suppl.): 1–138.
Nelson, J. S., T. C. Grande & M. V. H. Wilson. 2016. Fishes of the World 5th ed. Wiley.
Eriogonum spergulinum, the Spurry Buckwheat
Wandering around sandy highlands of the southwest United States, you may encounter a sparse, wiry weed growing between five and forty centimetres in height. This is the spurry buckwheat Eriogonum spergulinum.
Spurry buckwheat Eriogonum spergulinum, copyright Dcrjsr.
Members of the buckwheat family Polygonaceae are found worldwide but tend to be easily overlooked as low, scrubby weeds. In North America, one of the most diverse genera is Eriogonum, known from about 250 species though many are difficult to readily distinguish (Hickman 1993). Eriogonum spergulinum is one of the more recognisable species in the genus. As mentioned above, it grows in sandy soils, particularly those dominated by worn-down granite, and is found at altitudes between 1200 and 3500 metres. It is an annual herb with basal leaves of a linear shape, less than two millimetres wide but up to thirty millimetres long. The greater part of the plant's height is made up by the slender, cyme-like inflorescence bearing unribbed, four-toothed involucres on slender stalks. The flowers are up to three millimetres in diameter with a white perianth marked by darker stripes. Overall, E. spergulinum in flower resembles a drifting cloud of small white stars.
Close-up on Eriogonum spergulinum flowers, copyright Tom Hilton.
Three varieties of Eriogonum spergulinum have been recognised though they are not always distinct and tend to intergrade with each other. In most parts of the species' range, plants belong to the variety E. spergulinum var. reddingianum. This variety is characterised by erect inflorescences with glandular axes and flowers about two millimetres in diameter. The other two varieties are both restricted to the Sierra Nevada mountains of California. Eriogonum spergulinum var spergulinum resembles var. reddingianum but produces larger flowers, about three millimetres in diameter. Eriogonum spergulinum var. pratense is more distinctive. Inflorescences are prostrate to ascending, only about two to five millimetres in height, and lack glands on the axes. Flowers are only 1.5 millimetres across. Pratense is also a higher-altitude variety, found at heights above 2500 metres. The Sierra Nevada varieties are both uncommon; if any variety is likely to be found, it is the widespread reddingianum.
REFERENCE
Hickman, J. C. (ed.) 1993. The Jepson Manual: Higher Plants of California. University of California Press: Berkeley (California).
Members of the buckwheat family Polygonaceae are found worldwide but tend to be easily overlooked as low, scrubby weeds. In North America, one of the most diverse genera is Eriogonum, known from about 250 species though many are difficult to readily distinguish (Hickman 1993). Eriogonum spergulinum is one of the more recognisable species in the genus. As mentioned above, it grows in sandy soils, particularly those dominated by worn-down granite, and is found at altitudes between 1200 and 3500 metres. It is an annual herb with basal leaves of a linear shape, less than two millimetres wide but up to thirty millimetres long. The greater part of the plant's height is made up by the slender, cyme-like inflorescence bearing unribbed, four-toothed involucres on slender stalks. The flowers are up to three millimetres in diameter with a white perianth marked by darker stripes. Overall, E. spergulinum in flower resembles a drifting cloud of small white stars.
Three varieties of Eriogonum spergulinum have been recognised though they are not always distinct and tend to intergrade with each other. In most parts of the species' range, plants belong to the variety E. spergulinum var. reddingianum. This variety is characterised by erect inflorescences with glandular axes and flowers about two millimetres in diameter. The other two varieties are both restricted to the Sierra Nevada mountains of California. Eriogonum spergulinum var spergulinum resembles var. reddingianum but produces larger flowers, about three millimetres in diameter. Eriogonum spergulinum var. pratense is more distinctive. Inflorescences are prostrate to ascending, only about two to five millimetres in height, and lack glands on the axes. Flowers are only 1.5 millimetres across. Pratense is also a higher-altitude variety, found at heights above 2500 metres. The Sierra Nevada varieties are both uncommon; if any variety is likely to be found, it is the widespread reddingianum.
REFERENCE
Hickman, J. C. (ed.) 1993. The Jepson Manual: Higher Plants of California. University of California Press: Berkeley (California).
Scaleyness is Next to Diatom-ness
The last few decades have seen significant advances in our understanding of microbial diversity. Consistent improvements in available technologies and methods for study, both molecular and ultrastructural, have allowed researchers to look further and deeper than they ever could before. Not only have they identified taxa that were previously unknown, they have been able to develop a much better understanding of how microbial taxa relate to each other. Among the fields that has seen particularly remarkable advances has been the study of the picoplankton, that component of the marine plankton comprising organisms less than two or three microns in size. Much of the picoplankton, of course, is made up of bacteria but another significant component is species of microalgae belonging to the group known as heterokonts or stramenopiles.
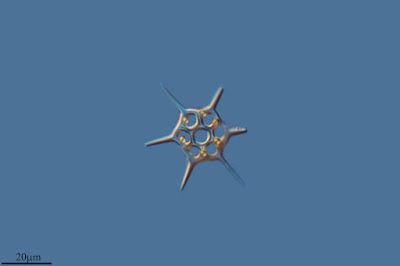
Schematic diagram of motile bolidophyte cell, from Guillou et al. (1999).
Heterokonts are a major clade of eukaryotes that are commonly characterised by cells bearing anterior pairs of morphologically distinct cilia. One of the cilia is longer and bears rows of hairs referred to as mastigonemes; the other, shorter cilium is usually smooth. Many heterokont species are photosynthetic and belong to a subclade of the heterokonts known as the ochrophytes. For most people, the best known ochrophytes will be the often-decidedly-not-microbial brown algae such as kelps. However, ochrophytes also include a broad diversity of microbial forms. Most ochrophyte cells share a characteristic golden-brown coloration owing to the presence of yellowish pigments such as fucoxanthin as well as the more standard chlorophyll.
Recent molecular studies have supported a division of the ochrophytes between two major clades. On one side are the brown algae and their closer microbial relatives. In the other clade are those ochrophytes more closely related to the diatoms. Appropriately enough, this latter clade was dubbed the Diatomista by Derelle et al. (2016). Other than the diatoms themselves, most representatives of the Diatomista belong to the picoplankton. For the most part, diatoms have lost the cilia otherwise associated with heterokonts. The only exceptions are the reproductive sperm cells which have a single anterior cilium bearing mastigonemes (Adl et al. 2019). The remaining Diatomista commonly have cells bearing one or two anterior cilia (if only one cilium is present, it will typically have mastigonemes). Nevertheless, the basal apparatus of the cilia is reduced, lacking microtubular roots or a rhizoplast, suggestive of an intermediate stage towards total loss (Guillou et al. 1999). Many also bear a covering of silica scales; enlargement of individual scales may have lead to the evolution of diatom-style frustules.
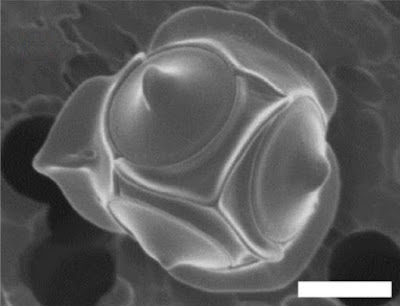
Non-motile cell of Triparma laevis f. inornata, from Kuwata et al. (1987).
The closest known relatives of diatoms are currently classified as the class Bolidophyceae. Motile cells of the Bolidophyceae were first described in 1999 (Guillou et al. 1999). They possessed two cilia, with the haired cilium directed anteriorly and the smooth cilium directed posteriorly, and lacked silica scales. Nevertheless, they were identified as the sister group to diatoms by molecular data. This was corroborated by the absence of a transitional helix structure at the base of each cilium, a feature shared with diatom sperm cells. Guillou et al. (1999) commented on the relatively high mobility of the bolidophytes, in contrast to the general expectation that picoplankton should exhibit a reduction in individual cell mobility owing to the difficulty in meeting energy demands.
The concept of bolidophytes shifted somewhat in the 2010s with the isolation in culture of the Parmales, a group of minute eukaryotes that had first been recognised in the 1980s but had long eluded detailed characterisation. These were non-motile cells enclosed within ornate silica scales. Once molecular data become available, researchers realised that 'Parmales' were not just closely related to 'bolidophytes', they were close enough that the two forms could reasonably be included in a single genus (Kuwata et al. 2018). The exact details of their connection, however, remain uncertain. It seems likely that the flagellate and non-flagellate forms represent alternate forms of single species. But whether we are looking at alternate generations of the life cycle, or whether the flagellate cells are generated in response to particular conditions, remains to be determined.
Skeleton of silicoflagellate Dictyocha speculum, copyright Proyecto Agua.
The remaining members of the Diatomista form a clade currently treated as including three classes, the Dictyochophyceae, Pelagophyceae and Pinguiophyceae. Together they are a diverse array of minute organisms, whether ciliated or amoeboid, naked or carrying organic scales, photosynthetic or heterotrophic or some combination of both. Among the representatives of the Dictyochophyceae are the so-called silicoflagellates, ciliated cells reinforced with a skeleton of (duh) silica. Though only a few species of silicoflagellate are recognised in the modern environment, they have an extensive fossil record extending back to the Middle Cretaceous (Kristiansen 1990). In some places, their preserved skeletons may dominate rock formations. Silicoflagellates appear to have reached their peak in the Miocene, followed by a decline to their modern condition. The exact interpretation of the silicoflagellate fossil record is a long-standing challenge (whether differences in morphology are taxonomic or environmental, for instance) but they hold the potential to tell us much about the history of our seas.
REFERENCES
Adl, S. M., D. Bass, C. E. Lane, J. Lukeš, C. L. Schoch, A. Smirnov, S. Agatha, C. Berney, M. W. Brown, F. Burki, P. Cárdenas, I. Čepička, L. Chistyakova, J. del Campo, M. Dunthorn, B. Edvardsen, Y. Eglit, L. Guillou, V. Hampl, A. A. Heiss, M. Hoppenrath, T. Y. James, A. Karnkowska, S. Karpov, E. Kim, M. Kolisko, A. Kudryavtsev, D. J. G. Lahr, E. Lara, L. Le Gall, D. H. Lynn, D. G. Mann, R. Massana, E. A. D. Mitchell, C. Morrow, J. S. Park, J. W. Pawlowski, M. J. Powell, D. J. Richter, S. Rueckert, L. Shadwick, S. Shimano, F. W. Spiegel, G. Torruella, N. Youssef, V. Zlatogursky & Q. Zhang. 2019. Revisions to the classification, nomenclature, and diversity of eukaryotes. Journal of Eukaryotic Microbiology 66: 4–119.
Derelle, R., P. López-García, H. Timpano & D. Moreira. 2016. A phylogenomic framework to study the diversity and evolution of stramenopiles (=heterokonts). Molecular Biology and Evolution 33 (11): 2890–2898.
Guillou, L., M.-J. Chrétiennot-Dinet, L. K. Medlin, H. Claustre, S. Loiseaux-de Goër & D. Vaulot. 1999. Bolidomonas: a new genus with two species belonging to a new algal class, the Bolidophyceae (Heterokonta). Journal of Phycology 35: 368–381.
Kristiansen, J. 1990. Phylum Chrysophyta. In: Margulis, L., J. O. Corliss, M. Melkonian & D. J. Chapman (eds) Handbook of Protoctista. The structure, cultivation, habitats and life histories of the eukaryotic microorganisms and their descendants exclusive of animals, plants and fungi. A guide to the algae, ciliates, foraminifera, sporozoa, water molds, slime molds and the other protoctists pp. 438–453. Jones & Bartlett Publishers: Boston. Kuwata, A., K. Yamada, M. Ichinomiya, S. Yoshikawa, M. Tragin, D. Vaulot & A. Lopes de Santos. 2018. Bolidophyceae, a sister picoplanktonic group of diatoms—a review. Frontiers in Marine Science 5: 370.
Heterokonts are a major clade of eukaryotes that are commonly characterised by cells bearing anterior pairs of morphologically distinct cilia. One of the cilia is longer and bears rows of hairs referred to as mastigonemes; the other, shorter cilium is usually smooth. Many heterokont species are photosynthetic and belong to a subclade of the heterokonts known as the ochrophytes. For most people, the best known ochrophytes will be the often-decidedly-not-microbial brown algae such as kelps. However, ochrophytes also include a broad diversity of microbial forms. Most ochrophyte cells share a characteristic golden-brown coloration owing to the presence of yellowish pigments such as fucoxanthin as well as the more standard chlorophyll.
Recent molecular studies have supported a division of the ochrophytes between two major clades. On one side are the brown algae and their closer microbial relatives. In the other clade are those ochrophytes more closely related to the diatoms. Appropriately enough, this latter clade was dubbed the Diatomista by Derelle et al. (2016). Other than the diatoms themselves, most representatives of the Diatomista belong to the picoplankton. For the most part, diatoms have lost the cilia otherwise associated with heterokonts. The only exceptions are the reproductive sperm cells which have a single anterior cilium bearing mastigonemes (Adl et al. 2019). The remaining Diatomista commonly have cells bearing one or two anterior cilia (if only one cilium is present, it will typically have mastigonemes). Nevertheless, the basal apparatus of the cilia is reduced, lacking microtubular roots or a rhizoplast, suggestive of an intermediate stage towards total loss (Guillou et al. 1999). Many also bear a covering of silica scales; enlargement of individual scales may have lead to the evolution of diatom-style frustules.
The closest known relatives of diatoms are currently classified as the class Bolidophyceae. Motile cells of the Bolidophyceae were first described in 1999 (Guillou et al. 1999). They possessed two cilia, with the haired cilium directed anteriorly and the smooth cilium directed posteriorly, and lacked silica scales. Nevertheless, they were identified as the sister group to diatoms by molecular data. This was corroborated by the absence of a transitional helix structure at the base of each cilium, a feature shared with diatom sperm cells. Guillou et al. (1999) commented on the relatively high mobility of the bolidophytes, in contrast to the general expectation that picoplankton should exhibit a reduction in individual cell mobility owing to the difficulty in meeting energy demands.
The concept of bolidophytes shifted somewhat in the 2010s with the isolation in culture of the Parmales, a group of minute eukaryotes that had first been recognised in the 1980s but had long eluded detailed characterisation. These were non-motile cells enclosed within ornate silica scales. Once molecular data become available, researchers realised that 'Parmales' were not just closely related to 'bolidophytes', they were close enough that the two forms could reasonably be included in a single genus (Kuwata et al. 2018). The exact details of their connection, however, remain uncertain. It seems likely that the flagellate and non-flagellate forms represent alternate forms of single species. But whether we are looking at alternate generations of the life cycle, or whether the flagellate cells are generated in response to particular conditions, remains to be determined.
The remaining members of the Diatomista form a clade currently treated as including three classes, the Dictyochophyceae, Pelagophyceae and Pinguiophyceae. Together they are a diverse array of minute organisms, whether ciliated or amoeboid, naked or carrying organic scales, photosynthetic or heterotrophic or some combination of both. Among the representatives of the Dictyochophyceae are the so-called silicoflagellates, ciliated cells reinforced with a skeleton of (duh) silica. Though only a few species of silicoflagellate are recognised in the modern environment, they have an extensive fossil record extending back to the Middle Cretaceous (Kristiansen 1990). In some places, their preserved skeletons may dominate rock formations. Silicoflagellates appear to have reached their peak in the Miocene, followed by a decline to their modern condition. The exact interpretation of the silicoflagellate fossil record is a long-standing challenge (whether differences in morphology are taxonomic or environmental, for instance) but they hold the potential to tell us much about the history of our seas.
REFERENCES
Adl, S. M., D. Bass, C. E. Lane, J. Lukeš, C. L. Schoch, A. Smirnov, S. Agatha, C. Berney, M. W. Brown, F. Burki, P. Cárdenas, I. Čepička, L. Chistyakova, J. del Campo, M. Dunthorn, B. Edvardsen, Y. Eglit, L. Guillou, V. Hampl, A. A. Heiss, M. Hoppenrath, T. Y. James, A. Karnkowska, S. Karpov, E. Kim, M. Kolisko, A. Kudryavtsev, D. J. G. Lahr, E. Lara, L. Le Gall, D. H. Lynn, D. G. Mann, R. Massana, E. A. D. Mitchell, C. Morrow, J. S. Park, J. W. Pawlowski, M. J. Powell, D. J. Richter, S. Rueckert, L. Shadwick, S. Shimano, F. W. Spiegel, G. Torruella, N. Youssef, V. Zlatogursky & Q. Zhang. 2019. Revisions to the classification, nomenclature, and diversity of eukaryotes. Journal of Eukaryotic Microbiology 66: 4–119.
Derelle, R., P. López-García, H. Timpano & D. Moreira. 2016. A phylogenomic framework to study the diversity and evolution of stramenopiles (=heterokonts). Molecular Biology and Evolution 33 (11): 2890–2898.
Guillou, L., M.-J. Chrétiennot-Dinet, L. K. Medlin, H. Claustre, S. Loiseaux-de Goër & D. Vaulot. 1999. Bolidomonas: a new genus with two species belonging to a new algal class, the Bolidophyceae (Heterokonta). Journal of Phycology 35: 368–381.
Kristiansen, J. 1990. Phylum Chrysophyta. In: Margulis, L., J. O. Corliss, M. Melkonian & D. J. Chapman (eds) Handbook of Protoctista. The structure, cultivation, habitats and life histories of the eukaryotic microorganisms and their descendants exclusive of animals, plants and fungi. A guide to the algae, ciliates, foraminifera, sporozoa, water molds, slime molds and the other protoctists pp. 438–453. Jones & Bartlett Publishers: Boston. Kuwata, A., K. Yamada, M. Ichinomiya, S. Yoshikawa, M. Tragin, D. Vaulot & A. Lopes de Santos. 2018. Bolidophyceae, a sister picoplanktonic group of diatoms—a review. Frontiers in Marine Science 5: 370.
Glyphyalinia Snails
North America (as with pretty much everywhere in the world outside the coldest regions) is home to a wide diversity of small, terrestrial snails that tend to pass unnoticed. Among the more diverse of these is the zonitid genus Glyphyalinia.
Glyphyalinia carolinensis, copyright John Slapcinsky.
Glyphyalinia species are often found in forest leaf-litter in the eastern part of North America. They have a low, translucent shell that is often about half a centimetre in diameter. Whorls of the shell increase regularly in size and are marked by a series of strongly impressed radiating lines in addition to finer growth lines. The umbilicus of the shell varies between species from completely absent to quite wide (Burch & Pearce 1990). The soft body of the animal varies in coloration, again depending on species. That of G. roemeri is all white except for the eyes; that of G. wheatleyi is almost uniformly black. The reproductive system of Glyphyalinia (which are hermaphroditic) includes a well-developed epiphallus and a distinct, ovoid spermathecal sac (Baker 1930).
Multiple species of Glyphyalinia may be found living in a single patch of forest though, at present, we know little about how (and whether) micro-habitats are partitioned between species. Some species seem to tolerate a wide variety of soil types and are correspondingly widely distributed. Others are more selective and localised; some may be considered endangered by habitat degradation. Even supposedly widespread species may be more vulnerable than appreciated: at least some may represent clusters of closely related species rather than truly uniform populations. These tiny snails can be notoriously difficult to study, making for a risk that they might just slip away barely noticed.
REFERENCES
Baker, H. B. 1930. The North American Retinellae. Proceedings of the Academy of Natural Sciences of Philadelphia 82: 193–219.
Burch, J. B., & T. A. Pearce. 1990. Terrestrial Gastropoda. In: Dindal, D. L. (ed.) Soil Biology Guide pp. 201–309. John Wiley & Sones: New York.
Glyphyalinia species are often found in forest leaf-litter in the eastern part of North America. They have a low, translucent shell that is often about half a centimetre in diameter. Whorls of the shell increase regularly in size and are marked by a series of strongly impressed radiating lines in addition to finer growth lines. The umbilicus of the shell varies between species from completely absent to quite wide (Burch & Pearce 1990). The soft body of the animal varies in coloration, again depending on species. That of G. roemeri is all white except for the eyes; that of G. wheatleyi is almost uniformly black. The reproductive system of Glyphyalinia (which are hermaphroditic) includes a well-developed epiphallus and a distinct, ovoid spermathecal sac (Baker 1930).
Multiple species of Glyphyalinia may be found living in a single patch of forest though, at present, we know little about how (and whether) micro-habitats are partitioned between species. Some species seem to tolerate a wide variety of soil types and are correspondingly widely distributed. Others are more selective and localised; some may be considered endangered by habitat degradation. Even supposedly widespread species may be more vulnerable than appreciated: at least some may represent clusters of closely related species rather than truly uniform populations. These tiny snails can be notoriously difficult to study, making for a risk that they might just slip away barely noticed.
REFERENCES
Baker, H. B. 1930. The North American Retinellae. Proceedings of the Academy of Natural Sciences of Philadelphia 82: 193–219.
Burch, J. B., & T. A. Pearce. 1990. Terrestrial Gastropoda. In: Dindal, D. L. (ed.) Soil Biology Guide pp. 201–309. John Wiley & Sones: New York.
Williamsita
A while back, I wrote a post about the crabronid wasp genus Podagritus. This time, I'm going to cover another crabronid genus found here in Australia: Williamsita.
Williamsita sp., copyright David Francis.
Like Podagritus, Williamsita species are boldly coloured wasps, typically mostly black with contrasting yellow or orange markings. They differ from Podagritus species in being more robust with the base of the gaster not notably pedunculate. Other distinguishing features include the presence of distinct foveae (pits) against the margins of the eyes (occasionally less distinct in males), thirteen-segmented antennae in males, and a pygidial plate in both sexes that is narrowed and concave in females, quadrate in males. Williamsita species also do not have the palps reduced as in Podagritus, instead having the more typical arrangement of six segments in the maxillary palps and four segments in the labial palps (Bohart & Menke 1976).
To date, eleven species have been recognised in the genus Williamsita (Leclercq 2006). Most are found in Australia with a single species each known from New Caledonia and Vanuatu. Leclercq (1950) suggested dividing the genus between two subgenera with all species except the New Caledonian type species W. novocaledonica forming a subgenus Androcrabro. Features supporting the latter taxon included the presence of ventral notches on one or more segments of the antennae in males. However, Leclercq later suggested abandoning such a formal division, questioning its significance (Leclercq 2006). The Australian species of Williamsita are, nevertheless, distinct from the two insular species in being marked with much stronger punctation over the body.
Most Williamsita species remain little seen and poorly known. However, breeding habits have been recorded for two Australian species, W. bivittata and W. tasmanica (Maynard & Fearn 2021; McCorquodale et al. 1989). Both these species nest in branching holes in rotting wood, either commandeering burrows left by wood-boring insects or excavating their own. Prey consists of larger flies such as blow flies or soldier flies which were carried back to the nest by the wasp running with the fly carried below the body. Up to six paralysed flies might be placed lying on their backs in a nest cell with an egg laid across the 'throat' (i.e. at the joint between head and thorax) of one of the flies. The cell would then be closed with a plug of woody frass. McCorquodale et al. (1989) recorded W. bivittata constructing several such cells in a series along a single tunnel, whereas Maynard & Fearn (2021) found W. tasmanica more likely to place a single cell in a side-branch. As both observations were limited to a single location in a single season, though, one might reasonably question whether these represent true differences in species behaviour or were determined by available conditions. There's a limit to how deep a Williamsita can burrow.
REFERENCES
Bohart, R. M., & A. S. Menke. 1976. Sphecid Wasps of the World. University of California Press: Berkeley.
Leclercq, J. 1950. Sur les crabroniens orientaux et australiens rangés par R. E. Turner (1912–1915) dans le genre Crabro (subgenus Solenius). Bulletin et Annales de la Société Entomologique de Belgique 86 (7–8): 191–198.
Leclercq, J. 2006. Hyménoptères crabroniens d'Australie du genre Williamsita Pate, 1947 (Hymenoptera: Crabronidae). Notes Fauniques de Gembloux 59 (2): 115–119.
Maynard, D., & S. Fearn. 2021. Ecological and behavioural observations of a nesting aggregation of the endemic Tasmanian digger wasp Williamsita tasmanica (Smith, 1856) (Hymenoptera: Crabronidae: Crabroninae). Papers and Proceedings of the Royal Society of Tasmania 155 (1): 43–50.
McCorquodale, D. B., C. E. Thomson & V. Elder. 1989. Nest and prey of Williamsita bivittata (Turner) (Hymenoptera: Sphecidae: Crabroninae). Australian Entomological Magazine 16 (1): 5–8.
Like Podagritus, Williamsita species are boldly coloured wasps, typically mostly black with contrasting yellow or orange markings. They differ from Podagritus species in being more robust with the base of the gaster not notably pedunculate. Other distinguishing features include the presence of distinct foveae (pits) against the margins of the eyes (occasionally less distinct in males), thirteen-segmented antennae in males, and a pygidial plate in both sexes that is narrowed and concave in females, quadrate in males. Williamsita species also do not have the palps reduced as in Podagritus, instead having the more typical arrangement of six segments in the maxillary palps and four segments in the labial palps (Bohart & Menke 1976).
To date, eleven species have been recognised in the genus Williamsita (Leclercq 2006). Most are found in Australia with a single species each known from New Caledonia and Vanuatu. Leclercq (1950) suggested dividing the genus between two subgenera with all species except the New Caledonian type species W. novocaledonica forming a subgenus Androcrabro. Features supporting the latter taxon included the presence of ventral notches on one or more segments of the antennae in males. However, Leclercq later suggested abandoning such a formal division, questioning its significance (Leclercq 2006). The Australian species of Williamsita are, nevertheless, distinct from the two insular species in being marked with much stronger punctation over the body.
Most Williamsita species remain little seen and poorly known. However, breeding habits have been recorded for two Australian species, W. bivittata and W. tasmanica (Maynard & Fearn 2021; McCorquodale et al. 1989). Both these species nest in branching holes in rotting wood, either commandeering burrows left by wood-boring insects or excavating their own. Prey consists of larger flies such as blow flies or soldier flies which were carried back to the nest by the wasp running with the fly carried below the body. Up to six paralysed flies might be placed lying on their backs in a nest cell with an egg laid across the 'throat' (i.e. at the joint between head and thorax) of one of the flies. The cell would then be closed with a plug of woody frass. McCorquodale et al. (1989) recorded W. bivittata constructing several such cells in a series along a single tunnel, whereas Maynard & Fearn (2021) found W. tasmanica more likely to place a single cell in a side-branch. As both observations were limited to a single location in a single season, though, one might reasonably question whether these represent true differences in species behaviour or were determined by available conditions. There's a limit to how deep a Williamsita can burrow.
REFERENCES
Bohart, R. M., & A. S. Menke. 1976. Sphecid Wasps of the World. University of California Press: Berkeley.
Leclercq, J. 1950. Sur les crabroniens orientaux et australiens rangés par R. E. Turner (1912–1915) dans le genre Crabro (subgenus Solenius). Bulletin et Annales de la Société Entomologique de Belgique 86 (7–8): 191–198.
Leclercq, J. 2006. Hyménoptères crabroniens d'Australie du genre Williamsita Pate, 1947 (Hymenoptera: Crabronidae). Notes Fauniques de Gembloux 59 (2): 115–119.
Maynard, D., & S. Fearn. 2021. Ecological and behavioural observations of a nesting aggregation of the endemic Tasmanian digger wasp Williamsita tasmanica (Smith, 1856) (Hymenoptera: Crabronidae: Crabroninae). Papers and Proceedings of the Royal Society of Tasmania 155 (1): 43–50.
McCorquodale, D. B., C. E. Thomson & V. Elder. 1989. Nest and prey of Williamsita bivittata (Turner) (Hymenoptera: Sphecidae: Crabroninae). Australian Entomological Magazine 16 (1): 5–8.
Opening Dors
My current dayjob mostly revolves around identifying and counting dung beetles. When Europeans settled Australia, they brought their farm animals with them. Unfortunately, the large piles of dung produced by cattle and horses proved rather daunting to native scavengers used to the more compact droppings of kangaroos and possums. And if you've ever experienced an Australian summer, you'll know that flies are definitely a thing. To help with this situation, Australia has had a long-running programme introducing exotic dung beetles that are better able to clean up after livestock. Most of these are members of the typical dung beetle family Scarabaeidae but one species, Geotrupes spiniger, represents a different subgroup of the superfamily Scarabaeoidea. These are the earth-boring dung beetles or dor beetles of the Geotrupidae.
Dor beetle Geotrupes spiniger, copyright Udo Schmidt.
The geotrupids are medium-sized to very large beetles, ranging in size from half a centimetre to 4.5 cm in length (Jameson 2002). Like many other members of the Scarabaeoidea, they have broad fore legs used for digging. Their short, eleven-segmented antennae end in the asymmetrical club typical of scarabaeoids but they may be distinguished from other families in that the basal segment of the three-segmented club is expanded to form a 'cup' against which the other segments may be tightly closed. The body of geotrupids is strongly convex, and is smooth and shiny dorsally but hairy underneath. In many species, the males may bear elaborate horns and/or processes on the head and pronotum.
Male Taurocerastes patagonicus, copyright Nicolás Lavandero.
Despite their size, geotrupids are secretive animals, spending most of their time in burrows underground (which may be up to three metres in depth) and usually only emerging at night. Various species feed on animal dung or decaying matter; some feed on subterranean fungi. In at least some species, eggs are laid in brood chambers within the parent's home burrow and multiple life stages may share a single burrow. Burrows may also be shared between multiple adults when conditions demand. Though adults do not directly tend to larvae, they may stock brood chambers with food supplies. In some Australian species of the subfamily Bolboceratinae, females lay a single gigantic egg at a time that may be up to 56% the size of its layer (Houston 2011). Larvae hatching from such an egg are able to develop right through to maturity without feeding.
Adult geotrupids produce a stridulating noise when disturbed which is the origin of the alternate vernacular name of "dor beetle" ("dor" being an old word for a buzzing insect). Larvae may or may not be capable of stridulation, depending on the species.
Male Blackburnium rhinoceros, copyright Edward Bell.
The classification of geotrupids is the subject of ongoing investigation. A recent classification divides the family between three subfamilies, the widespread Geotrupinae and Bolboceratinae and the South American Taurocerastinae. Morphological differences between these subfamilies, particularly at the larval stage, have lead some researchers to question whether the Geotrupidae in the broad sense represents a monophyletic group. Molecular analyses thus far seem ambiguous; an analysis by McKenna et al. (2015) placed geotrupids as part of a polytomy near the base of the scarabaeoids. As an aside, my supervisor recently asked myself and a retired colleague whether Geotrupes spiniger was the only species of geotrupid found in Australia. I replied "yes", our colleague responded "no". Our conflict, of course, was based on whether Australia's wide diversity of Bolboceratinae contributed to the count.
REFERENCES
Houston, T. F. 2011. Egg gigantism in some Australian earth-borer beetles (Coleoptera: Geotrupidae: Bolboceratinae) and its apparent association with reduction or elimination of larval feeding. Australian Journal of Entomology 50: 164–173.
Jameson, M. L. 2002. Geotrupidae Latreille 1802. In: Arnett, R. H., Jr, M. C. Thomas, P. E. Skelley & J. H. Frank (eds) American Beetles vol. 2. Polyphaga: Scarabaeoidea through Curculionoidea pp. 23–27. CRC Press.
McKenna, D. D., B. D. Farrell, M. S. Caterino, C. W. Farnum, D. C. Hawks, D. R. Maddison, A. E. Seago, A. E. Z. Short, A. F. Newton & M. K. Thayer. 2015. Phylogeny and evolution of Staphyliniformia and Scarabaeiformia: forest litter as a stepping stone for diversification of nonphytophagous beetles. Systematic Entomology 40: 35–60.
The geotrupids are medium-sized to very large beetles, ranging in size from half a centimetre to 4.5 cm in length (Jameson 2002). Like many other members of the Scarabaeoidea, they have broad fore legs used for digging. Their short, eleven-segmented antennae end in the asymmetrical club typical of scarabaeoids but they may be distinguished from other families in that the basal segment of the three-segmented club is expanded to form a 'cup' against which the other segments may be tightly closed. The body of geotrupids is strongly convex, and is smooth and shiny dorsally but hairy underneath. In many species, the males may bear elaborate horns and/or processes on the head and pronotum.
Despite their size, geotrupids are secretive animals, spending most of their time in burrows underground (which may be up to three metres in depth) and usually only emerging at night. Various species feed on animal dung or decaying matter; some feed on subterranean fungi. In at least some species, eggs are laid in brood chambers within the parent's home burrow and multiple life stages may share a single burrow. Burrows may also be shared between multiple adults when conditions demand. Though adults do not directly tend to larvae, they may stock brood chambers with food supplies. In some Australian species of the subfamily Bolboceratinae, females lay a single gigantic egg at a time that may be up to 56% the size of its layer (Houston 2011). Larvae hatching from such an egg are able to develop right through to maturity without feeding.
Adult geotrupids produce a stridulating noise when disturbed which is the origin of the alternate vernacular name of "dor beetle" ("dor" being an old word for a buzzing insect). Larvae may or may not be capable of stridulation, depending on the species.
The classification of geotrupids is the subject of ongoing investigation. A recent classification divides the family between three subfamilies, the widespread Geotrupinae and Bolboceratinae and the South American Taurocerastinae. Morphological differences between these subfamilies, particularly at the larval stage, have lead some researchers to question whether the Geotrupidae in the broad sense represents a monophyletic group. Molecular analyses thus far seem ambiguous; an analysis by McKenna et al. (2015) placed geotrupids as part of a polytomy near the base of the scarabaeoids. As an aside, my supervisor recently asked myself and a retired colleague whether Geotrupes spiniger was the only species of geotrupid found in Australia. I replied "yes", our colleague responded "no". Our conflict, of course, was based on whether Australia's wide diversity of Bolboceratinae contributed to the count.
REFERENCES
Houston, T. F. 2011. Egg gigantism in some Australian earth-borer beetles (Coleoptera: Geotrupidae: Bolboceratinae) and its apparent association with reduction or elimination of larval feeding. Australian Journal of Entomology 50: 164–173.
Jameson, M. L. 2002. Geotrupidae Latreille 1802. In: Arnett, R. H., Jr, M. C. Thomas, P. E. Skelley & J. H. Frank (eds) American Beetles vol. 2. Polyphaga: Scarabaeoidea through Curculionoidea pp. 23–27. CRC Press.
McKenna, D. D., B. D. Farrell, M. S. Caterino, C. W. Farnum, D. C. Hawks, D. R. Maddison, A. E. Seago, A. E. Z. Short, A. F. Newton & M. K. Thayer. 2015. Phylogeny and evolution of Staphyliniformia and Scarabaeiformia: forest litter as a stepping stone for diversification of nonphytophagous beetles. Systematic Entomology 40: 35–60.
Platybunus: the Wide-Eyed Harvestmen of Europe
The western Palaearctic region (that is, Europe and the immediately adjacent parts of Asia and northern Africa) is home to a diverse and distinctive fauna of harvestmen. Among the various genera unique to this part of the world are the forest- and mountain-dwellers of the genus Platybunus.
Platybunus pinetorum, copyright Donald Hobern.
Platybunus species are moderate-sized long-legged harvestmen of the family Phalangiidae, the central body in larger individuals being about eight millimetres long (Martens 1978). Their most characteristic feature is a relatively large eye-mound, distinctly wider than long and occupying a large section of the anterior carapace. As with other European phalangiids, they eye-mound is ornamented with a row of denticles each side though the body lacks denticles over the remainder of the dorsum. The body is often comparatively slender, tapering towards the rear (particularly in males), and is marked on the dorsum by a darker median band. The pedipalps have a pair of well-developed setose apophyses on the inner distal ends of the patella and tibia, and a series of long spine-like tubercles on the underside of the femur. These tubercles presumably function in the capture of prey, forming a basket that can be closed around the harvestman's victims. External sexual dimorphism in Platybunus is fairly minimal though females are overall larger and fatter. The penis is notably long and slender with a relatively small glans, offset from the shaft by a more or less marked constriction.
Platybunus bucephalus, copyright Adrian Tync.
Martens (1978) recognises four species of Platybunus found in higher altitude regions of central Europe with the species P. bucephalus and P. pinetorum occupying much of the genus' range. Platybunus bucephalus may be distinguished from P. pinetorum by, among other features, its relatively shorter legs. Platybunus pallidus is endemic to the Carpathians, and the tiny P. alpinorelictus inhabits the Garda Mountains of northern Italy. Another species, P. anatolicus, was described from Turkey by Roewer (1956)*. In general, Platybunus species inhabit alpine and subalpine forests, being found among the herbaceous undergrowth, under bark or on rock faces. Where their ranges overlap, P. bucephalus is more accustomed to extending beyond the forest margins than P. pinetorum and may be found above the tree-line. In recent years, the range of P. pinetorum has extended northwards, being first recorded from the UK in 2010 and Sweden in 2015 (Fritzén et al. 2015). At least some populations of P. pinetorum are capable of reproducing parthenogenetically and this may have played a part in its spread.
*Platybunus mirus was described by Loman (1892) on the basis of two male specimens that supposedly came from Sumatra. Though the identity of this species has never been resolved (Loman's illustration of the penis is at least suggestive of a true Platybunus), the claimed locality seems almost certain to be an error of some kind.
The internal classification of the Phalangiidae remains in need of further investigation. Platybunus has been recognised by some authors as forming a subfamily Platybuninae with a cluster of other western Palaearctic genera bearing similar ventrally spined pedipalps (Zhang & Zhang 2012). However, other authors have not separated this group from the subfamily Phalangiinae. The platybunines may represent a phylogenetically coherent grouping, or their shared features may reflect adaptations to a similar life style. The genital morphology of Platybunus is recognisably distinct from that of other platybunines which may argue against any relationship (Martens 1978). On the other hand, platybunines might possibly be distinguished from phalangiines by the chemical composition of their repugnatorial gland secretions (Raspotnig et al. 2015). A formal analysis of the family's evolution would be a welcome advance.
REFERENCES
Fritzén, N. R., V. Rinne, M. Sunhede, A. Uddström, S. Van de Poel & P. De Smedt. 2015. Platybunus pinetorum (Arachnida, Opiliones) new to Sweden. Memoranda Soc. Fauna Flora Fennica 91: 37–40.
Loman, J. C. C. 1892. Opilioniden von Sumatra, Java und Flores. In: M. Weber (ed.) Zoologische Ergebnisse einer Reise in Niederländisch Ost-Indien vol. 3 pp. 1–26, pl. 1. E. J. Brill: Leiden.
Martens, J. 1978. Spinnentiere, Arachnida: Weberknechte, Opiliones. Gustav Fischer Verlag: Jena.
Raspotnig, G., M. Schaider, P. Föttinger, V. Leutgeb & C. Komposch. 2015. Benzoquinones from scent glands of phalangiid harvestmen (Arachnida, Opiliones, Eupnoi): a lesson from Rilaena triangularis. Chemoecology 25: 63–72.
Roewer, C. F. 1956. Über Phalangiinae (Phalangiidae, Opiliones Palpatores). (Weitere Weberknechte XIX). Senckenbergiana Biologica 37 (3–4): 247–318.
Zhang, C., & F. Zhang. 2012. On the subfamilial assignment of Platybunoides (Opiliones: Eupnoi: Phalangiidae), with the description of a new species from China. Zootaxa 3190: 47–55.
Platybunus species are moderate-sized long-legged harvestmen of the family Phalangiidae, the central body in larger individuals being about eight millimetres long (Martens 1978). Their most characteristic feature is a relatively large eye-mound, distinctly wider than long and occupying a large section of the anterior carapace. As with other European phalangiids, they eye-mound is ornamented with a row of denticles each side though the body lacks denticles over the remainder of the dorsum. The body is often comparatively slender, tapering towards the rear (particularly in males), and is marked on the dorsum by a darker median band. The pedipalps have a pair of well-developed setose apophyses on the inner distal ends of the patella and tibia, and a series of long spine-like tubercles on the underside of the femur. These tubercles presumably function in the capture of prey, forming a basket that can be closed around the harvestman's victims. External sexual dimorphism in Platybunus is fairly minimal though females are overall larger and fatter. The penis is notably long and slender with a relatively small glans, offset from the shaft by a more or less marked constriction.
Martens (1978) recognises four species of Platybunus found in higher altitude regions of central Europe with the species P. bucephalus and P. pinetorum occupying much of the genus' range. Platybunus bucephalus may be distinguished from P. pinetorum by, among other features, its relatively shorter legs. Platybunus pallidus is endemic to the Carpathians, and the tiny P. alpinorelictus inhabits the Garda Mountains of northern Italy. Another species, P. anatolicus, was described from Turkey by Roewer (1956)*. In general, Platybunus species inhabit alpine and subalpine forests, being found among the herbaceous undergrowth, under bark or on rock faces. Where their ranges overlap, P. bucephalus is more accustomed to extending beyond the forest margins than P. pinetorum and may be found above the tree-line. In recent years, the range of P. pinetorum has extended northwards, being first recorded from the UK in 2010 and Sweden in 2015 (Fritzén et al. 2015). At least some populations of P. pinetorum are capable of reproducing parthenogenetically and this may have played a part in its spread.
*Platybunus mirus was described by Loman (1892) on the basis of two male specimens that supposedly came from Sumatra. Though the identity of this species has never been resolved (Loman's illustration of the penis is at least suggestive of a true Platybunus), the claimed locality seems almost certain to be an error of some kind.
The internal classification of the Phalangiidae remains in need of further investigation. Platybunus has been recognised by some authors as forming a subfamily Platybuninae with a cluster of other western Palaearctic genera bearing similar ventrally spined pedipalps (Zhang & Zhang 2012). However, other authors have not separated this group from the subfamily Phalangiinae. The platybunines may represent a phylogenetically coherent grouping, or their shared features may reflect adaptations to a similar life style. The genital morphology of Platybunus is recognisably distinct from that of other platybunines which may argue against any relationship (Martens 1978). On the other hand, platybunines might possibly be distinguished from phalangiines by the chemical composition of their repugnatorial gland secretions (Raspotnig et al. 2015). A formal analysis of the family's evolution would be a welcome advance.
REFERENCES
Fritzén, N. R., V. Rinne, M. Sunhede, A. Uddström, S. Van de Poel & P. De Smedt. 2015. Platybunus pinetorum (Arachnida, Opiliones) new to Sweden. Memoranda Soc. Fauna Flora Fennica 91: 37–40.
Loman, J. C. C. 1892. Opilioniden von Sumatra, Java und Flores. In: M. Weber (ed.) Zoologische Ergebnisse einer Reise in Niederländisch Ost-Indien vol. 3 pp. 1–26, pl. 1. E. J. Brill: Leiden.
Martens, J. 1978. Spinnentiere, Arachnida: Weberknechte, Opiliones. Gustav Fischer Verlag: Jena.
Raspotnig, G., M. Schaider, P. Föttinger, V. Leutgeb & C. Komposch. 2015. Benzoquinones from scent glands of phalangiid harvestmen (Arachnida, Opiliones, Eupnoi): a lesson from Rilaena triangularis. Chemoecology 25: 63–72.
Roewer, C. F. 1956. Über Phalangiinae (Phalangiidae, Opiliones Palpatores). (Weitere Weberknechte XIX). Senckenbergiana Biologica 37 (3–4): 247–318.
Zhang, C., & F. Zhang. 2012. On the subfamilial assignment of Platybunoides (Opiliones: Eupnoi: Phalangiidae), with the description of a new species from China. Zootaxa 3190: 47–55.
Voley, Voley, Voley
Over a third of all living mammal species are rodents. In cooler regions of the Northern Hemisphere, the rodent fauna is often dominated by the Microtinae, the group of mouse-like rodents including voles and lemmings. And in North America, the most widespread of all microtine species is the eastern meadow vole Microtus pennsylvanicus.
Eastern meadow vole Microtus pennsylvanicus, copyright Gilles Gonthier.
The eastern meadow vole is found over most of Canada and a large part of the northern and eastern United States, with the subspecies M. p. chihuahuensis known from Chihuahua in northern Mexico. This species is about the size of a small rat, being from 14 to 20 cm in length with about three to six centimentres of that length being tail (Reich 1981). They are generally yellowish-brown in colour with black tips on the hairs though individuals vary significantly in brightness and shade. Western populations are supposed to be lighter in coloration than eastern, and southern individuals tend to be larger than northern. As an indication of this species' variability, Reich (1981) recognised 28 recognised subspecies.
Eastern meadow voles are primarily inhabitants of grasslands, with a preference for damper habitats, though they may also be found in woodlands. They mostly live in burrows underground, emerging to the surface to forage for food. Eastern meadow voles are generalist feeders, browsing on most available forms of low vegetation: grasses, sedges and herbs. When populations reach their peak, they may cause significant damage to woody plants by ringbarking their trunks. Individuals may seemingly be active at just about any time of day.
Eastern meadow vole in a state of danger, copyright David Allen.
Like other small rodents, meadow voles are short-lived animals with estimates of average lifespan ranging from just two or three months to ten to fourteen months (Reich 1981). Studies of movement patterns indicate that mature females generally maintain distinct, non-overlapping ranges whereas males range further and with less concern for others (Madison 1980). Mating behaviour appears generally promiscuous: males will range over the territories of multiple females and litters with mixed paternity are not uncommon (Boonstra et al. 1993). Paternal behaviour has been observed among eastern meadow voles in laboratory populations but all indications are that wild males do not remain with females after mating. Males often bear wounds indicative of intra-species conflict. These may be the result of males fighting over access to females but Madison (1980) suggested a potential alternative. Less dominant males might be more likely to attempt to approach females earlier or later in their oestrus cycle as the females are more likely to be guarded by dominant males when at their peak. While avoiding attacks from their dominant brethren, these minor males might find themselves violently rebuffed by a female who is just not yet in the mood.
After mating, gestation lasts for about three weeks, usually resulting in a litter of four to six babies. Weaning then takes place after about two weeks. Females forage far less while lactating than at other times. It might seem counter-intuitive for a female to reduce feeding when her energy demands are presumably at their peak but again Madison (1980) suggests an explanation: perhaps her energy needs are such that she simply lacks the capacity for extensive wandering. Young may potentially remain with their mother for some time after weaning but eventually they will be forced out of the parental burrow, leaving to face the wide world on their own. And when you're the size of a vole, that's a very wide world indeed.
REFERENCES
Boonstra, R., X. Xia & L. Pavone. 1993. Mating system of the meadow vole, Microtus pennsylvanicus. Behavioral Ecology 4: 83–89.
Madison, D. M. 1980. Space use and social structure in meadow voles, Microtus pennsylvanicus. Behavioral Ecology and Sociobiology 7: 65–71.
Reich, L. M. 1981. Microtus pennsylvanicus. Mammalian Species 159: 1–8.
The eastern meadow vole is found over most of Canada and a large part of the northern and eastern United States, with the subspecies M. p. chihuahuensis known from Chihuahua in northern Mexico. This species is about the size of a small rat, being from 14 to 20 cm in length with about three to six centimentres of that length being tail (Reich 1981). They are generally yellowish-brown in colour with black tips on the hairs though individuals vary significantly in brightness and shade. Western populations are supposed to be lighter in coloration than eastern, and southern individuals tend to be larger than northern. As an indication of this species' variability, Reich (1981) recognised 28 recognised subspecies.
Eastern meadow voles are primarily inhabitants of grasslands, with a preference for damper habitats, though they may also be found in woodlands. They mostly live in burrows underground, emerging to the surface to forage for food. Eastern meadow voles are generalist feeders, browsing on most available forms of low vegetation: grasses, sedges and herbs. When populations reach their peak, they may cause significant damage to woody plants by ringbarking their trunks. Individuals may seemingly be active at just about any time of day.
Like other small rodents, meadow voles are short-lived animals with estimates of average lifespan ranging from just two or three months to ten to fourteen months (Reich 1981). Studies of movement patterns indicate that mature females generally maintain distinct, non-overlapping ranges whereas males range further and with less concern for others (Madison 1980). Mating behaviour appears generally promiscuous: males will range over the territories of multiple females and litters with mixed paternity are not uncommon (Boonstra et al. 1993). Paternal behaviour has been observed among eastern meadow voles in laboratory populations but all indications are that wild males do not remain with females after mating. Males often bear wounds indicative of intra-species conflict. These may be the result of males fighting over access to females but Madison (1980) suggested a potential alternative. Less dominant males might be more likely to attempt to approach females earlier or later in their oestrus cycle as the females are more likely to be guarded by dominant males when at their peak. While avoiding attacks from their dominant brethren, these minor males might find themselves violently rebuffed by a female who is just not yet in the mood.
After mating, gestation lasts for about three weeks, usually resulting in a litter of four to six babies. Weaning then takes place after about two weeks. Females forage far less while lactating than at other times. It might seem counter-intuitive for a female to reduce feeding when her energy demands are presumably at their peak but again Madison (1980) suggests an explanation: perhaps her energy needs are such that she simply lacks the capacity for extensive wandering. Young may potentially remain with their mother for some time after weaning but eventually they will be forced out of the parental burrow, leaving to face the wide world on their own. And when you're the size of a vole, that's a very wide world indeed.
REFERENCES
Boonstra, R., X. Xia & L. Pavone. 1993. Mating system of the meadow vole, Microtus pennsylvanicus. Behavioral Ecology 4: 83–89.
Madison, D. M. 1980. Space use and social structure in meadow voles, Microtus pennsylvanicus. Behavioral Ecology and Sociobiology 7: 65–71.
Reich, L. M. 1981. Microtus pennsylvanicus. Mammalian Species 159: 1–8.
Anchor Sponges
Sponges are, by their very nature, a challenging group taxonomically. At the macroscopic level, they are often amorphous and indeterminate in appearance. As one taxonomist complained in 1842 (as quoted in Hooper & Van Soest 2002): "there is so much that is in common to them, and each adapts itself so readily to circumstances and assumes a new mask, that it requires a tact, to be gained only by some experience, to recognize them under their guises; while we labour, perhaps in vain, to devise phrases which shall aptly portray to others the characteristics of objects that have no fixed shape, and whose distinctive peculiarities almost cheat the eye". Reliable identification typically requires the close examination of microscopic details, in particular the conformation and arrangement of the mineralised spicules that make up the skeleton of many sponges.
Myxilla incrustans, copyright B. E. Picton.
The Myxillidae are a family of marine sponges that, so far as we currently know, are most diverse in temperate and frigid waters. Like other members of the class Demospongiae, the most diverse of the recognised sponge classes, they have a skeleton of spicules constructed from silica. Different arrangements of spicules allow the body of the sponge to be divided into two layers. In the outer ectosoma, which can be thought of as the 'skin' of the sponge, elongate spicules are vertically radiating or placed in 'bouquet' arrangements with a palisade of vertical spicules surmounted by radiating clusters. These spicules generally have each end similar and may be smooth or spiky. In the inner choanosoma, within which are placed the feeding chambers of the sponge, elongate spicules are placed in a reticulate arrangement. These spicules generally have one end pointed and the other blunt.
Skeletal arrangements and individual spicules from various Myxillidae, from Hooper & Van Soest (2002).
Mixed in amongst these larger megasclere spicules are smaller microscleres that do not form part of the main structural skeleton, though presumably they do help hold the sponge body together. In myxillids, the microscleres generally take the form of anchorate chelae, small curved structures with incurved rounded prongs at each end. Members of the boreal genus Melonanchora have a mixture of chelae and a different type of microsclere shaped like a ribbed rugby ball (Santín et al. 2021). In the Indo-West Pacific genus Psammochela, growing sponges will also incorporate sand from the surrounding environment to supplement the microscleres (de Voogd 2012).
Growth habit of Myxillidae can vary from encrusting to massive to branching. The species Stelodoryx procera, found around the Azores, has a distinctive growth habit with a flattened main body at the end of an elongate stalk. On the whole, though individual species may be distinguished by growth habit, species within a single genus may differ greatly in form. For determining genera, examination of spicules is really the only way to go.
REFERENCES
Hooper, J. N. A., & R. W. M. Van Soest. 2002. Systema Porifera: A guide to the classification of sponges vol. 1. Kluwer Academic/Plenum Publishers.
Santín, A., M.-J. Uriz, J. Cristobo, J. R. Xavier & P. Ríos. 2021. Unique spicules may confound species differentiation: taxonomy and biogeography of Melonanchora Carter, 1874 and two new related genera (Myxillidae: Poecilosclerida) from the Okhotsk Sea. PeerJ 9: e12515.
Voogd, N. J. de. 2012. On sand-bearing myxillid sponges, with a description of Psammochela tutiae sp. nov. (Poecilosclerida, Myxillina) from the northern Moluccas, Indonesia. Zootaxa 3155: 21–28.
The Myxillidae are a family of marine sponges that, so far as we currently know, are most diverse in temperate and frigid waters. Like other members of the class Demospongiae, the most diverse of the recognised sponge classes, they have a skeleton of spicules constructed from silica. Different arrangements of spicules allow the body of the sponge to be divided into two layers. In the outer ectosoma, which can be thought of as the 'skin' of the sponge, elongate spicules are vertically radiating or placed in 'bouquet' arrangements with a palisade of vertical spicules surmounted by radiating clusters. These spicules generally have each end similar and may be smooth or spiky. In the inner choanosoma, within which are placed the feeding chambers of the sponge, elongate spicules are placed in a reticulate arrangement. These spicules generally have one end pointed and the other blunt.
Mixed in amongst these larger megasclere spicules are smaller microscleres that do not form part of the main structural skeleton, though presumably they do help hold the sponge body together. In myxillids, the microscleres generally take the form of anchorate chelae, small curved structures with incurved rounded prongs at each end. Members of the boreal genus Melonanchora have a mixture of chelae and a different type of microsclere shaped like a ribbed rugby ball (Santín et al. 2021). In the Indo-West Pacific genus Psammochela, growing sponges will also incorporate sand from the surrounding environment to supplement the microscleres (de Voogd 2012).
Growth habit of Myxillidae can vary from encrusting to massive to branching. The species Stelodoryx procera, found around the Azores, has a distinctive growth habit with a flattened main body at the end of an elongate stalk. On the whole, though individual species may be distinguished by growth habit, species within a single genus may differ greatly in form. For determining genera, examination of spicules is really the only way to go.
REFERENCES
Hooper, J. N. A., & R. W. M. Van Soest. 2002. Systema Porifera: A guide to the classification of sponges vol. 1. Kluwer Academic/Plenum Publishers.
Santín, A., M.-J. Uriz, J. Cristobo, J. R. Xavier & P. Ríos. 2021. Unique spicules may confound species differentiation: taxonomy and biogeography of Melonanchora Carter, 1874 and two new related genera (Myxillidae: Poecilosclerida) from the Okhotsk Sea. PeerJ 9: e12515.
Voogd, N. J. de. 2012. On sand-bearing myxillid sponges, with a description of Psammochela tutiae sp. nov. (Poecilosclerida, Myxillina) from the northern Moluccas, Indonesia. Zootaxa 3155: 21–28.
Succulent Orchids
With over 1200 known species found in Asia and Australasia, Dendrobium is one of the largest currently recognised genera of orchids. As with other examples of such 'super-genera', the question of how to best handle such a monster has been fiercely debated. In 2003, Australian botanist M. Clements proposed dividing Dendrobium between numerous segregate genera, noting (among other reasons) that the genus as previously recognised was not monophyletic. However, Clements' system does not seem to have garnered widespread usage with other orchid systematists preferring to retain a broad concept of Dendrobium (excluding some of the more egregious outliers) that largely corresponds with its established usage (e.g. Schuiteman 2011). Nevertheless, many of the subdivisions promoted by Clements remain recognised as well delimited groups. One such cluster is the assemblage of species recognised as Dendrobium section Aporum.
Growth habit of Dendrobium sect. Aporum, copyright Tony Rodd.
Species of section Aporum are epiphytes found in lowland forests of south-east Asia, extending eastwards to New Guinea and the Solomon Islands. Members of this section have thin stems that are erect at first but tend to become pendulous as they lengthen. Leaves are fleshy and equitant: that is, they are folded longitudinally with what would otherwise be the two sides of the dorsal surface fused, except at the base where they overlap with opposing leaves. The stem may be more or less completely concealed by the leaf bases. Tips of the leaves end in a point. Flowers are borne singly or in clusters, arising laterally on the stem between leaf nodes or at the tip of the stem alongside a terminal scale. The flowers may be subtended by persistent chaffy bracts. They are generally small and fleshy and tend to be short-lived, wilting after just a few days.
Flowers of Dendrobium anceps, copyright Aqiao HQ.
The functional significance of the Aporum section's distinctive leaves remains uncertain. As noted by Carlsward et al. (1997), the fleshy leaves might be taken as an adaptation to water retention. However, though access to water is a consistent concern for epiphytes, the humid rainforests in which Aporum species are found hardly seem the driest of places. Conversely, the effective even distribution of stomata on both sides of leaf resulting from their equitant condition may make it easier for excess water to be released from the plant.
Dendrobium distichum, photographed by Ronny Boos.
Orchids in general are, of course, most often considered by people as ornamental plants. My impression is that the various Aporum species tend not to be among the most widely grown of species though their unusual growth habit might attract interest. This may be due to them not being the easiest of orchids to maintain; they appear to require high humidity and warm temperatures to thrive with a cooler, drier period in the non-growing season. Among the more popular species are Dendrobium anceps and D. keithii, both of which produce small greenish flowers. Those of D. anceps have been described as having a distinct "apple pie" fragrance. Of course, if you happen to be wandering through the jungles of south-east Asia, you might well discover these plants growing of their own accord.
REFERENCES
Carlsward, B. S., W. L. Stern, W. S. Judd & T. W. Lucansky. 1997. Comparative leaf anatomy and systematics in Dendrobium, sections Aporum and Rhizobium (Orchidaceae). International Journal of Plant Sciences 158 (3): 332–342.
Clements, M. A. 2003. Molecular phylogenetic systematics in the Dendrobiinae (Orchidaceae), with emphasis on Dendrobium section Pedilonum. Telopea 10 (1): 247–298.
Schuiteman, A. 2011. Dendrobium (Orchidaceae): to split or not to split? Gardens' Bulletin Singapore 63 (1–2): 245–257.
Species of section Aporum are epiphytes found in lowland forests of south-east Asia, extending eastwards to New Guinea and the Solomon Islands. Members of this section have thin stems that are erect at first but tend to become pendulous as they lengthen. Leaves are fleshy and equitant: that is, they are folded longitudinally with what would otherwise be the two sides of the dorsal surface fused, except at the base where they overlap with opposing leaves. The stem may be more or less completely concealed by the leaf bases. Tips of the leaves end in a point. Flowers are borne singly or in clusters, arising laterally on the stem between leaf nodes or at the tip of the stem alongside a terminal scale. The flowers may be subtended by persistent chaffy bracts. They are generally small and fleshy and tend to be short-lived, wilting after just a few days.
The functional significance of the Aporum section's distinctive leaves remains uncertain. As noted by Carlsward et al. (1997), the fleshy leaves might be taken as an adaptation to water retention. However, though access to water is a consistent concern for epiphytes, the humid rainforests in which Aporum species are found hardly seem the driest of places. Conversely, the effective even distribution of stomata on both sides of leaf resulting from their equitant condition may make it easier for excess water to be released from the plant.
Orchids in general are, of course, most often considered by people as ornamental plants. My impression is that the various Aporum species tend not to be among the most widely grown of species though their unusual growth habit might attract interest. This may be due to them not being the easiest of orchids to maintain; they appear to require high humidity and warm temperatures to thrive with a cooler, drier period in the non-growing season. Among the more popular species are Dendrobium anceps and D. keithii, both of which produce small greenish flowers. Those of D. anceps have been described as having a distinct "apple pie" fragrance. Of course, if you happen to be wandering through the jungles of south-east Asia, you might well discover these plants growing of their own accord.
REFERENCES
Carlsward, B. S., W. L. Stern, W. S. Judd & T. W. Lucansky. 1997. Comparative leaf anatomy and systematics in Dendrobium, sections Aporum and Rhizobium (Orchidaceae). International Journal of Plant Sciences 158 (3): 332–342.
Clements, M. A. 2003. Molecular phylogenetic systematics in the Dendrobiinae (Orchidaceae), with emphasis on Dendrobium section Pedilonum. Telopea 10 (1): 247–298.
Schuiteman, A. 2011. Dendrobium (Orchidaceae): to split or not to split? Gardens' Bulletin Singapore 63 (1–2): 245–257.
The Huenellidae
Researchers who deal with the modern marine fauna are used to thinking of brachiopods as a marginal group, their diversity greatly overshadowed on a global scale by the superficially similar bivalves. However, modern brachiopods are but a shadow of their former selves; for much of the Palaeozoic era, their relationship with the bivalves was the inverse of today. Many are the brachiopod lineages that came and went over this time.
External views of ventral (left) and dorsal valves of Huenella triplicata, from Walcott (1924).
The Huenellidae were an assemblage of brachiopods that lived during the late Cambrian and early Ordovician (Amsden & Biernat 1965). They represent early representatives of the Pentamerida, a Palaeozoic order of fairly generalised-looking brachiopods. Within the Pentamerida, they fall within the suborder Syntrophiidina. Syntrophiidinans as a whole are rarely found in the fossil record and as a result remain poorly known. Members of the suborder share a distinctive shape with biconvex valves marked by a dorsal fold and ventral sulcus. That is, the midline of the shell is raised above either side with the ventral valve forming a 'valley' to match the raised 'hill' of the dorsal valve. What, if anything, was the purpose of this arrangement I wouldn't know but modern brachiopods often inhabit locations with a lot of organic silt and/or fine sediment. Perhaps the uneven level of the syntrophiidinan shell helped protect it from burial by a shifting substrate.
Interior view of ventral valve of Radkeina taylori, from Laurie (1997), with scoop-shaped spondylium at upper midline.
Families of Syntrophiidina may be distinguished based on the development of the spondylium, an internal projection at the base of the ventral valve that provided an attachment site for the shell muscles. Members of the Huenellidae possessed either a sessile spondylium or a pseudospondylium, a spondylium-type structure rising from the internal surface of the valve itself rather than from the hinge. Amsden & Biernat (1965) recognised a division of the huenellids between two subfamilies based on the development of the brachiophore plates, projections on the inside of the dorsal valve that would have supported the lophophore. Members of the Huenellinae possessed more developed brachiophores than members of the Mesonomiinae. Outer ornament of the huenellid shell varied from more or less smooth with weak concentric ridges to costate with distinct radiating ridges.
Phylogenetic relationships within the Syntrophiidina do not seem to have been established in detail but the early appearance in the fossil record of huenellids at least raises the question of whether they included the ancestors of later families. As well as other families of the Syntrophiidina, candidates for descent would include members of the suborder Pentameridina as well as of the related order Rhynchonellida. This latter order includes species which survive to the present day so the possibility exists that while the huenellids themselves may be long gone, their legacy may yet live on.
REFERENCE
Amsden, T. W., & G. Biernat. 1965. Pentamerida. In: Moore, R. C. (ed.) Treatise on Invertebrate Paleontology pt H. Brachiopoda vol. 2 pp. H523–H552. The Geological Society of America, Inc.: Boulder (Colorado), and The University of Kansas Press: Lawrence (Kansas).
The Huenellidae were an assemblage of brachiopods that lived during the late Cambrian and early Ordovician (Amsden & Biernat 1965). They represent early representatives of the Pentamerida, a Palaeozoic order of fairly generalised-looking brachiopods. Within the Pentamerida, they fall within the suborder Syntrophiidina. Syntrophiidinans as a whole are rarely found in the fossil record and as a result remain poorly known. Members of the suborder share a distinctive shape with biconvex valves marked by a dorsal fold and ventral sulcus. That is, the midline of the shell is raised above either side with the ventral valve forming a 'valley' to match the raised 'hill' of the dorsal valve. What, if anything, was the purpose of this arrangement I wouldn't know but modern brachiopods often inhabit locations with a lot of organic silt and/or fine sediment. Perhaps the uneven level of the syntrophiidinan shell helped protect it from burial by a shifting substrate.
Families of Syntrophiidina may be distinguished based on the development of the spondylium, an internal projection at the base of the ventral valve that provided an attachment site for the shell muscles. Members of the Huenellidae possessed either a sessile spondylium or a pseudospondylium, a spondylium-type structure rising from the internal surface of the valve itself rather than from the hinge. Amsden & Biernat (1965) recognised a division of the huenellids between two subfamilies based on the development of the brachiophore plates, projections on the inside of the dorsal valve that would have supported the lophophore. Members of the Huenellinae possessed more developed brachiophores than members of the Mesonomiinae. Outer ornament of the huenellid shell varied from more or less smooth with weak concentric ridges to costate with distinct radiating ridges.
Phylogenetic relationships within the Syntrophiidina do not seem to have been established in detail but the early appearance in the fossil record of huenellids at least raises the question of whether they included the ancestors of later families. As well as other families of the Syntrophiidina, candidates for descent would include members of the suborder Pentameridina as well as of the related order Rhynchonellida. This latter order includes species which survive to the present day so the possibility exists that while the huenellids themselves may be long gone, their legacy may yet live on.
REFERENCE
Amsden, T. W., & G. Biernat. 1965. Pentamerida. In: Moore, R. C. (ed.) Treatise on Invertebrate Paleontology pt H. Brachiopoda vol. 2 pp. H523–H552. The Geological Society of America, Inc.: Boulder (Colorado), and The University of Kansas Press: Lawrence (Kansas).
Arranging Nautiloids
For years, the higher taxonomy of cephalopods was expressed as a division between three subclasses: the Nautiloidea, the Ammonoidea and the Coleoidea. Coleoids were the clade of cephalopods that had lost the external shell, ammonoids were a Mesozoic lineage with complex septa dividing the chambers of the shell, and nautiloids were... the rest. From the tiny, possibly benthic, curved cones of the Cambrian where the class began, to gigantic straight-shelled monsters of the later Palaeozoic, to the modern chambered nautilus, all were lumped together as 'nautiloids'. The nautiloid subclass was explicitly understood to include the ancestors of the others but recognition of more phylogenetically coherent subgroups has been hampered by poor understanding about how the various nautiloid lineages were interrelated. And part of the problem in this regard has been uncertainty about just what features of their fossils we should be paying attention to.
Diorama reconstruction of Beloitoceras oncocerids, from the Burpee Museum.
One factor that has drawn attention in recent years has been the arrangement of muscle scars on the shell. Large muscle attachment scars appear as raised annular elevations on the inside of the shell towards the rear end of the body chamber (in practice, they are more often observed in fossils as depressions on the internal mould). In the living nautilus, the muscles attached to these scars function in the retraction of the head (King & Evans 2019). Modern nautilus possess a pair of large lateral scars in an arrangement that has been labelled 'pleuromyarian'. However, many of the earliest cephalopods possessed a ring of numerous small scars, an arrangement referred to as 'oncomyarian'. Other cephalopods might have scars restricted to the dorsal ('dorsomyarian') or ventral ('ventromyarian') midline.
Primary types of muscle scar in nautiloids, from King & Evans (2019). 'D' and 'V' indicate dorsal and ventral, respectively, and arrows indicate direction of aperture.
Another feature that has been called out has been the structure of the connecting rings around the siphuncle. Shelled cephalopods, you will recall, have the shell divided into chambers separated by septa. Though the bulk of the animal is found in the final body chamber, a fleshy cord called the siphuncle runs back through the remaining chambers. In life, the siphuncle is used to control the levels of fluid in the chambers, which in turn controls the animal's buoyancy. The boundary between the siphuncle and the surrounding chamber is marked a toughened sheath, referred to as the connecting ring. In the modern nautilus, the connecting ring is comprised of two layers, an outer calcareous layer and an inner chitinous layer. In comparable fossils, the latter chitinous layer has decomposed after death so only the outer layer is preserved. However, some extinct cephalopod groups preserve evidence of calcification in the inner as well as the outer layer. Based on the distinction between these two siphuncle types, Mutvei (2015) supported dividing most of the nautiloids between two major lineages, the Nautilosiphonata (with a nautilus-type siphuncle) and the Calciosiphonata (with the internally calcified connecting rings).
A couple of years earlier, the same author (Mutvei 2013) had proposed recognition of a superorder Multiceratoidea for nautiloids that combined multiple muscle scars with a nautilus-type siphuncle. Examples of nautiloid orders with such a combination included the Ellesmeroceratida (small nautiloids with densely placed septa), the Oncoceratida (often short, squat nautiloids) and the Discosorida (similarly squat forms with complex bulging connecting rings). All of these were found in the earlier part of the Palaeozoic with the oncoceratids dieing off in the early Carboniferous. Mutvei (2013) also included the coiled Tarphyceratida and the egg-shaped Ascoceratida in this group. Later, King & Evans (2019) redefined this grouping as the Multiceratia, excluding the Tarphyceratida and Ascoceratida on the grounds that they had ventromyarian rather than oncomyarian muscle scars. Mutvei (2013) suggested that, rather than representing retractor muscles, these smaller repeated scars were associated with an outgrowth of the mantle, either as tentacles or a muscular 'skirt', that was used to capture micro-plankton.
Phylogeny of 'nautiloids' supported by King & Evans (2019). Though not shown on this diagram, the majority of authors have suggested that ammonoids and coleoids are descended from Orthoceratida.
King & Evans (2019) proposed a reclassification of the subclass Nautiloidea between five subclasses defined primarily by muscle structure. Apart from the earliest oncomyarian Plectronoceratia, most 'nautiloids' could be divided between two lineages. On one side were the dorsomyarian Orthoceratia (usually thought to include the ancestors of the ammonoids and coleoids). On the other, the oncomyarian Multiceratia would eventually give rise to the ventromyarian Tarphyceratia which in turn included the ancestors of the pleuromyarian Nautilida. Note that many of the reocognised subclasses (and orders) remain paraphyletic but we are at least approaching a more informative picture of cephalopod evolution than the earlier unceremonious dumping into 'Nautiloidea' (I should probably also remind you that, for various reasons, most invertebrate palaeontologists still don't regard strict monophyly as a taxonomic requirement in and of itself).
The usage of muscle scars and connecting rings as classificatory keys is handicapped by the difficulty of observing them. As internal structures, they each require careful preparation of a specimen to observe. And once you've gotten to a position where you can see them, it seems not to be particularly easy to tell just what you're looking at. As a result, muscle scarring and siphon structure remains undescribed for the majority of nautiloid species. Judging the structure of connecting rings seems to be particularly challenging and some have gone so far as to suggest that purported different structures may be the result of post-mortem taphonomic processes (King & Evans 2019). Nevertheless, what we do know suggests that such features remain reasonably consistent within each of the well-recognised nautiloid orders. And Mutvei's (2015) concept of Calciosiphonata vs Nautilosiphonata does largely line up with King & Evans' (2019) dorsomyarian vs oncomyarian-ventromyarian lineages. There are, of course, some notable exceptions. Whether these will cause the developing structure to collapse, or whether they indicate mistakes in interpretation, only continued research will tell.
REFERENCES
King, A. H., & D. H. Evans. 2019. High-level classification of the nautiloid cephalopods: a proposal for the revision of the Treatise Part K. Swiss Journal of Palaeontology 138: 65–85.
Mutvei, H. 2013. Characterization of nautiloid orders Ellesmerocerida, Oncocerida, Tarphycerida, Discosorida and Ascocerida: new superorder Multiceratoidea. GFF 135 (2): 171–183.
Mutvei, H. 2015. Characterization of two new superorders Nautilosiphonata and Calciosiphonata and a new order Cyrtocerinida of the subclass Nautiloidea; siphuncular structure in the Ordovician nautiloid Bathmoceras (Cephalopoda). GFF 137 (3): 164–174.
One factor that has drawn attention in recent years has been the arrangement of muscle scars on the shell. Large muscle attachment scars appear as raised annular elevations on the inside of the shell towards the rear end of the body chamber (in practice, they are more often observed in fossils as depressions on the internal mould). In the living nautilus, the muscles attached to these scars function in the retraction of the head (King & Evans 2019). Modern nautilus possess a pair of large lateral scars in an arrangement that has been labelled 'pleuromyarian'. However, many of the earliest cephalopods possessed a ring of numerous small scars, an arrangement referred to as 'oncomyarian'. Other cephalopods might have scars restricted to the dorsal ('dorsomyarian') or ventral ('ventromyarian') midline.
Another feature that has been called out has been the structure of the connecting rings around the siphuncle. Shelled cephalopods, you will recall, have the shell divided into chambers separated by septa. Though the bulk of the animal is found in the final body chamber, a fleshy cord called the siphuncle runs back through the remaining chambers. In life, the siphuncle is used to control the levels of fluid in the chambers, which in turn controls the animal's buoyancy. The boundary between the siphuncle and the surrounding chamber is marked a toughened sheath, referred to as the connecting ring. In the modern nautilus, the connecting ring is comprised of two layers, an outer calcareous layer and an inner chitinous layer. In comparable fossils, the latter chitinous layer has decomposed after death so only the outer layer is preserved. However, some extinct cephalopod groups preserve evidence of calcification in the inner as well as the outer layer. Based on the distinction between these two siphuncle types, Mutvei (2015) supported dividing most of the nautiloids between two major lineages, the Nautilosiphonata (with a nautilus-type siphuncle) and the Calciosiphonata (with the internally calcified connecting rings).
A couple of years earlier, the same author (Mutvei 2013) had proposed recognition of a superorder Multiceratoidea for nautiloids that combined multiple muscle scars with a nautilus-type siphuncle. Examples of nautiloid orders with such a combination included the Ellesmeroceratida (small nautiloids with densely placed septa), the Oncoceratida (often short, squat nautiloids) and the Discosorida (similarly squat forms with complex bulging connecting rings). All of these were found in the earlier part of the Palaeozoic with the oncoceratids dieing off in the early Carboniferous. Mutvei (2013) also included the coiled Tarphyceratida and the egg-shaped Ascoceratida in this group. Later, King & Evans (2019) redefined this grouping as the Multiceratia, excluding the Tarphyceratida and Ascoceratida on the grounds that they had ventromyarian rather than oncomyarian muscle scars. Mutvei (2013) suggested that, rather than representing retractor muscles, these smaller repeated scars were associated with an outgrowth of the mantle, either as tentacles or a muscular 'skirt', that was used to capture micro-plankton.
King & Evans (2019) proposed a reclassification of the subclass Nautiloidea between five subclasses defined primarily by muscle structure. Apart from the earliest oncomyarian Plectronoceratia, most 'nautiloids' could be divided between two lineages. On one side were the dorsomyarian Orthoceratia (usually thought to include the ancestors of the ammonoids and coleoids). On the other, the oncomyarian Multiceratia would eventually give rise to the ventromyarian Tarphyceratia which in turn included the ancestors of the pleuromyarian Nautilida. Note that many of the reocognised subclasses (and orders) remain paraphyletic but we are at least approaching a more informative picture of cephalopod evolution than the earlier unceremonious dumping into 'Nautiloidea' (I should probably also remind you that, for various reasons, most invertebrate palaeontologists still don't regard strict monophyly as a taxonomic requirement in and of itself).
The usage of muscle scars and connecting rings as classificatory keys is handicapped by the difficulty of observing them. As internal structures, they each require careful preparation of a specimen to observe. And once you've gotten to a position where you can see them, it seems not to be particularly easy to tell just what you're looking at. As a result, muscle scarring and siphon structure remains undescribed for the majority of nautiloid species. Judging the structure of connecting rings seems to be particularly challenging and some have gone so far as to suggest that purported different structures may be the result of post-mortem taphonomic processes (King & Evans 2019). Nevertheless, what we do know suggests that such features remain reasonably consistent within each of the well-recognised nautiloid orders. And Mutvei's (2015) concept of Calciosiphonata vs Nautilosiphonata does largely line up with King & Evans' (2019) dorsomyarian vs oncomyarian-ventromyarian lineages. There are, of course, some notable exceptions. Whether these will cause the developing structure to collapse, or whether they indicate mistakes in interpretation, only continued research will tell.
REFERENCES
King, A. H., & D. H. Evans. 2019. High-level classification of the nautiloid cephalopods: a proposal for the revision of the Treatise Part K. Swiss Journal of Palaeontology 138: 65–85.
Mutvei, H. 2013. Characterization of nautiloid orders Ellesmerocerida, Oncocerida, Tarphycerida, Discosorida and Ascocerida: new superorder Multiceratoidea. GFF 135 (2): 171–183.
Mutvei, H. 2015. Characterization of two new superorders Nautilosiphonata and Calciosiphonata and a new order Cyrtocerinida of the subclass Nautiloidea; siphuncular structure in the Ordovician nautiloid Bathmoceras (Cephalopoda). GFF 137 (3): 164–174.
Subscribe to:
Comments (Atom)